Evaluation of the Long-Term Outcomes with Everolimus After Calcineurin Inhibitor Withdrawal: 36M Results of the H2304 and PROTECT Extension Studies.
1H2304 study group, Hamburg, Germany
2PROTECT study group, Hamburg, Germany
3Novartis Pharmaceuticals Corporation, East Hanover, NJ
4Novartis Pharma AG, Basel, Switzerland.
Meeting: 2016 American Transplant Congress
Abstract number: 144
Keywords: Efficacy, Immunosuppression, Liver transplantation, Renal function
Session Information
Session Name: Concurrent Session: Liver: Immunosuppression and Rejection
Session Type: Concurrent Session
Date: Sunday, June 12, 2016
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:30pm-5:42pm
Presentation Time: 5:30pm-5:42pm
Location: Room 312
Background: The H2304 and PROTECT studies demonstrated reduced nephrotoxicity with everolimus (EVR)-based calcineurin inhibitor (CNI; cyclosporine: CsA, tacrolimus: TAC)-free regimens at Month (M) 12. Here, we evaluate the approaches based on 36M results from CNI withdrawal (WD) vs standard (C) CNI regimens from these studies.
Methods: H2304 study recruited patients in CNI-WD (N = 231) arm to receive EVR (C0 3-8 ng/mL; increased to C0 6-10 ng/mL by end of M4) + rTAC (C0 3-5 ng/mL; withdrawn at M4), 1M post-liver transplantation (LTx). Enrollment into CNI-WD arm was prematurely terminated due to higher acute rejection rate during CNI withdrawal; however, patients on study treatment for >4M could continue in the regimen. In PROTECT, patients in CNI-WD (N = 101) arm received EVR + CNI (TAC or CsA) 4-8 weeks post-LTx with EVR C0 target of 5-12 when combined with TAC or 8-12 ng/mL in combination with CsA. After CNI withdrawal EVR C0 was maintained at 5-12 ng/mL.CNI was completely withdrawn when patients were stable with 70% CNI reduction (for at least 2M) latest by M6 post-LTx and all patients received basiliximab induction therapy.
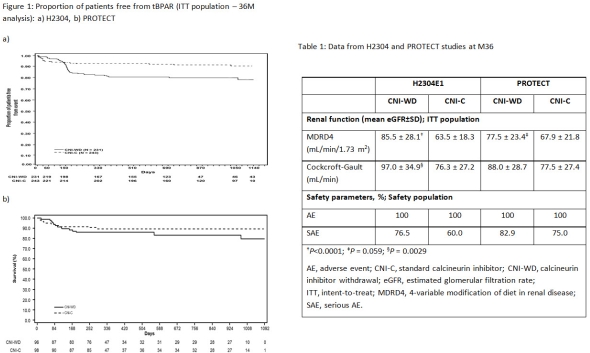
Results: At M36, incidence of tBPAR was higher in CNI-WD arm vs CNI-C in both studies (Figure 1a & 1b). However, there was no increase in graft loss in CNI-WD vs CNI-C arm (H2304: 2.8% vs 4.0%; PROTECT: 2.2% vs 2.1%). In both the studies, renal function (eGFR; MDRD4) improved significantly in CNI-WD vs CNI-C arms and incidence of AEs and SAEs was similar in CNI-WD vs CNI-C arm (Table 1).
Conclusion: Although an increased risk of rejection was seen at the time of CNI withdrawal in H2304 study, complete CNI withdrawal without risk of subsequent efficacy failure, can be achieved with the introduction of induction therapy and stepwise CNI reduction as seen in PROTECT. Despite the differences in rejection rates, CNI-WD arm in both studies showed better renal function preservation vs CNI-C arm.
CITATION INFORMATION: Fischer L, Fung J, Metselaar H, Kaiser G, Schemmer P, Neuhaus P, Dong G, Lopez P, Bernhardt P, Schlitt H. Evaluation of the Long-Term Outcomes with Everolimus After Calcineurin Inhibitor Withdrawal: 36M Results of the H2304 and PROTECT Extension Studies. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Fischer L, Fung J, Metselaar H, Kaiser G, Schemmer P, Neuhaus P, Dong G, Lopez P, Bernhardt P, Schlitt H. Evaluation of the Long-Term Outcomes with Everolimus After Calcineurin Inhibitor Withdrawal: 36M Results of the H2304 and PROTECT Extension Studies. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/evaluation-of-the-long-term-outcomes-with-everolimus-after-calcineurin-inhibitor-withdrawal-36m-results-of-the-h2304-and-protect-extension-studies/. Accessed March 9, 2026.« Back to 2016 American Transplant Congress
