Evaluating Long Term Outcomes in Kidney Transplant Patients with TruGraf Gene Expression and TRAC Dd-cfdna in the TRULO Study
Eurofins Transplant Genomics, Framingham, MA
Meeting: 2022 American Transplant Congress
Abstract number: 1582
Keywords: Gene expression, Kidney transplantation, Outcome, Rejection
Topic: Basic Science » Basic Clinical Science » 17 - Biomarkers: Clinical Outcomes
Session Information
Session Name: Biomarkers: Clinical Outcomes
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
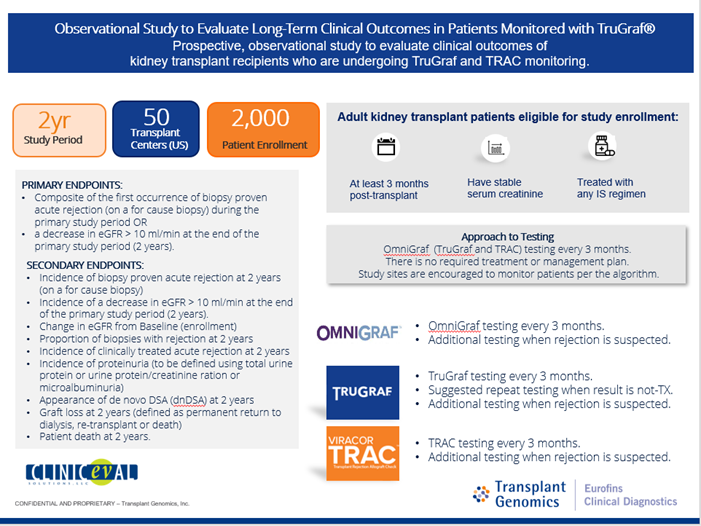
*Purpose: The aim of this study is to evaluate long term clinical outcomes in kidney transplant patients undergoing non-invasive rejection monitoring with the OmniGraf® biomarker panel (TruGraf® and TRAC™)
*Methods: The TRULO (TruGraf Long-term Clinical Outcomes) study is a prospective, multi-center, observational study evaluating quarterly surveillance and additional testing when rejection is suspected (for cause) with the OmniGraf biomarker panel, which includes both TruGraf® Gene Expression Profile and TRAC™ donor-derived cell free DNA (dd-cfDNA). The TruGraf test is a qualitative, “rule out” assay that confirms if a patient’s peripheral gene expression is similar to a biopsy confirmed phenotype of no rejection (immune quiescence). TRAC™ dd-cfDNA utilizes single nucleotide polymorphisms (SNPs) present in genes of the organ donor compared to the recipient to estimate the fraction of circulating cfDNA originating from donor. Recent data support that utilizing a combination of gene expression and dd-cfDNA may be complementary in aiding the diagnosis of subclinical rejection.
*Results: The TRULO study will collect data over a 2-year study period with 2000 subjects at about 50 US transplant centers. The primary end point is a composite of the occurrence of either biopsy proven acute rejection (on a for cause biopsy) OR a decrease from baseline in eGFR>10ml/min at 2-years post-enrollment. A key secondary endpoint is the evaluation of the clinical utility surveys performed with each set of TruGraf and TRAC results.
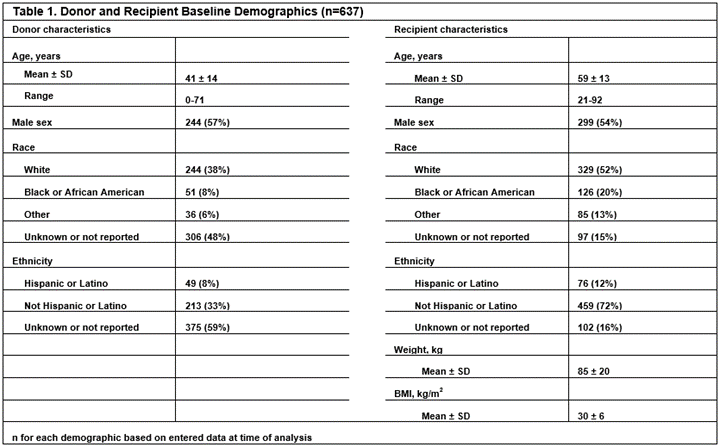
*Conclusions: Enrollment is competitive and site recruitment is still in process with Table 1 describing baseline demographics of the 637 enrolled subjects at time of analysis.
To cite this abstract in AMA style:
West-Thielke P, Ally W, Hickey J, Stach L, Fleming J, Cober T, Kawano A, Agboli I, Miller C, Holman J. Evaluating Long Term Outcomes in Kidney Transplant Patients with TruGraf Gene Expression and TRAC Dd-cfdna in the TRULO Study [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/evaluating-long-term-outcomes-in-kidney-transplant-patients-with-trugraf-gene-expression-and-trac-dd-cfdna-in-the-trulo-study/. Accessed March 9, 2026.« Back to 2022 American Transplant Congress