Efficacy and Safety of Three Different Treatment Regimen in De Novo Renal Transplant Patients: Month 48 Follow-Up Results of the HERAKLES Trial
1Herakles Study Group, Germany
2Novartis Pharma, Nuremberg, Germany
3Herakles Study Group, Switzerland.
Meeting: 2015 American Transplant Congress
Abstract number: 210
Keywords: Immunosuppression, Kidney transplantation, Multicenter studies, Safety
Session Information
Session Name: Concurrent Session: Kidney: Immunosupression Minimization
Session Type: Concurrent Session
Date: Monday, May 4, 2015
Session Time: 2:15pm-3:45pm
 Presentation Time: 3:03pm-3:15pm
Presentation Time: 3:03pm-3:15pm
Location: Room 113-BC
Aim: To compare safety and efficacy of 3 different immunosuppressive (IS) regimen 4years after renal transplantation (Tx).
Methods: 802 patients (pts) were included in this 1year, prospective, open-label, randomized (rdz), controlled multi-center study with observational follow-up (FU) until Mo60 post Tx. After induction therapy all pts received cyclosporine A (CsA), enteric-coated mycophenolate sodium (EC-MPS) and steroids. 3months (Mo) post Tx 499pts were rdz 1:1:1 to either a) continue standard CsA(100-180ng/ml)+EC-MPS(n=166) (STD) or convert b) to a calcineurin inhibitor (CNI)-free regimen with everolimus (EVR)(5-10ng/ml)+EC-MPS(n=171) or c) to a CNI-low regimen with EVR(3-8ng/ml) + reduced CsA(50-75ng/ml) (n=162). All pts continued on steroids. At time of Mo48 FU interims-analysis data were available from 110 (73%) STD, 117(79%) CNI-free and 111(76%) CNI-low treated pts of the FU ITT population (pop).
Results: From rdz to Mo48 BPAR was reported in 19/151(13%)STD, 24/149(16%)CNI-free and in 23/147(16%)CNI-low pts (ITT;p=ns). 5 deaths (3%) occurred in STD, 3 (2%) in CNI-free and 6 (4%) in the CNI-low group. 9 (6%) graft losses were observed in the STD, 6(4%) in the CNI-free and 2(1%) in the CNI-low group. Composite failure (BPAR,death,graft loss,loss to FU) occured in 32‚ (21 %) STD, 36 (24%) CNI-free, 39(27%) CNI-low treated pts. Premature discontinuation due to AEs occurred in 5 (3%) of STD, 5 (3%) of CNI-free and 1 (1%) of CNI-low pts (safety-pop) from Mo12 to 48. Renal function (cGFR, Nankivell, LOCF) was significantly improved by +6.8mL/min/1.73m2 in favor of the CNI-free regimen at Mo48 (ITT;p=0.02). 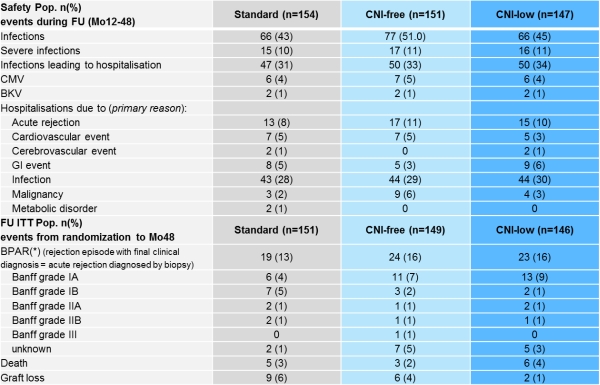
Conclusions: Mo48 results from HERAKLES show that IS regimen using EVR with low-dose or without CNI-exposure reflect an efficacious and safe therapeutic approach offering the opportunity for an individualized IS to minimize CNI-exposure.
To cite this abstract in AMA style:
Sommerer C, Budde K, Kliem V, Witzke O, Guba M, Jacobi J, Vogt B, Hauser I, Reinke P, Stahl R, Rath T, Porstner M, Baeumer D, Zeier M, Arns W, Lehner F. Efficacy and Safety of Three Different Treatment Regimen in De Novo Renal Transplant Patients: Month 48 Follow-Up Results of the HERAKLES Trial [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/efficacy-and-safety-of-three-different-treatment-regimen-in-de-novo-renal-transplant-patients-month-48-follow-up-results-of-the-herakles-trial/. Accessed June 30, 2025.« Back to 2015 American Transplant Congress
