Efficacy and Safety of Delayed, Low-Dose Valganciclovir in High-Risk Cytomegalovirus Liver Transplant Recipients
1Ochsner Medical Center, New Orleans, LA, 2Xavier University, New Orleans, LA
Meeting: 2022 American Transplant Congress
Abstract number: 1664
Keywords: Cytomeglovirus, Economics, Liver transplantation, Outcome
Topic: Clinical Science » Pharmacy » 30 - Non-Organ Specific: Clinical Pharmacy/Transplant Pharmacotherapy
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: The goal of this study was to compare the safety and efficacy of a delayed valganciclovir (VGCV) dosing protocol for cytomegalovirus (CMV) prophylaxis in liver transplant (LT) recipients who are high risk for CMV (D+/R-).
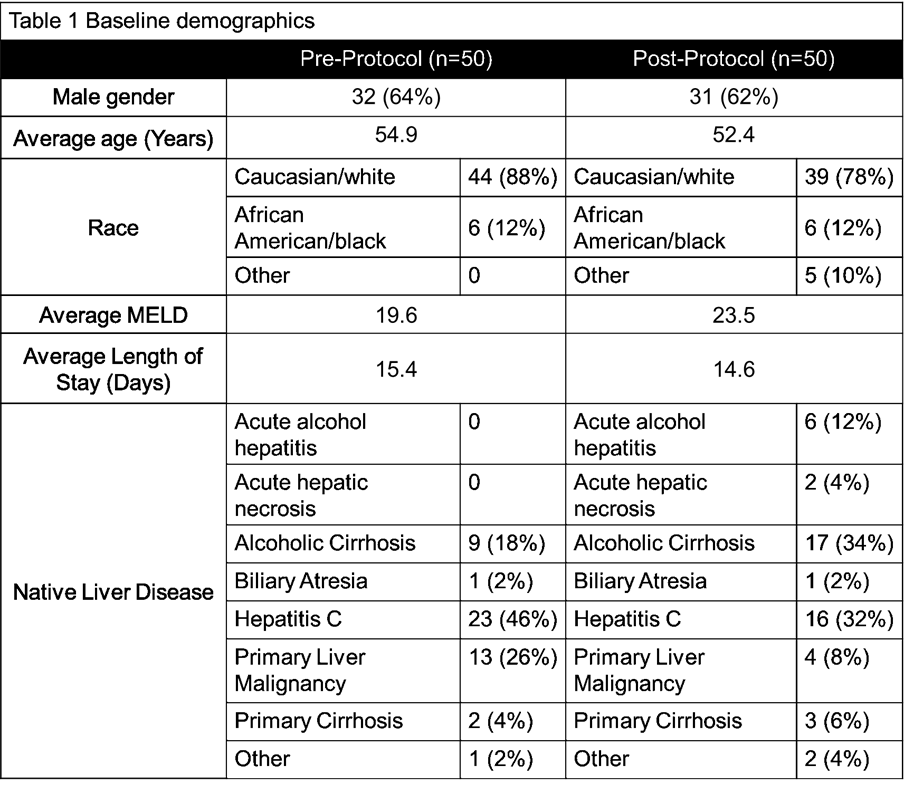
*Methods: This was a single-center, retrospective review of adult CMV high risk LT recipients that evaluated a 4/2019 protocol change that delayed the initiation of VGCV. All CMV high risk recipients received VGCV 450mg/day (reduced to TIW for CrCl <40) for 180 days, which was initiated on post-op day (POD) 0 in the pre-protocol group and on POD 10 or hospital discharge in the post-protocol group. The primary outcome was CMV disease at 1 year. Secondary outcomes included tissue-invasive disease, breakthrough CMV infection, and detection of CMV resistant genotypes. Safety outcomes included neutropenia (defined as an absolute neutrophil count < 750), growth colony stimulating factors use (G-CSF), biopsy-proven rejection (BPAR), graft loss, and death at 1 year.
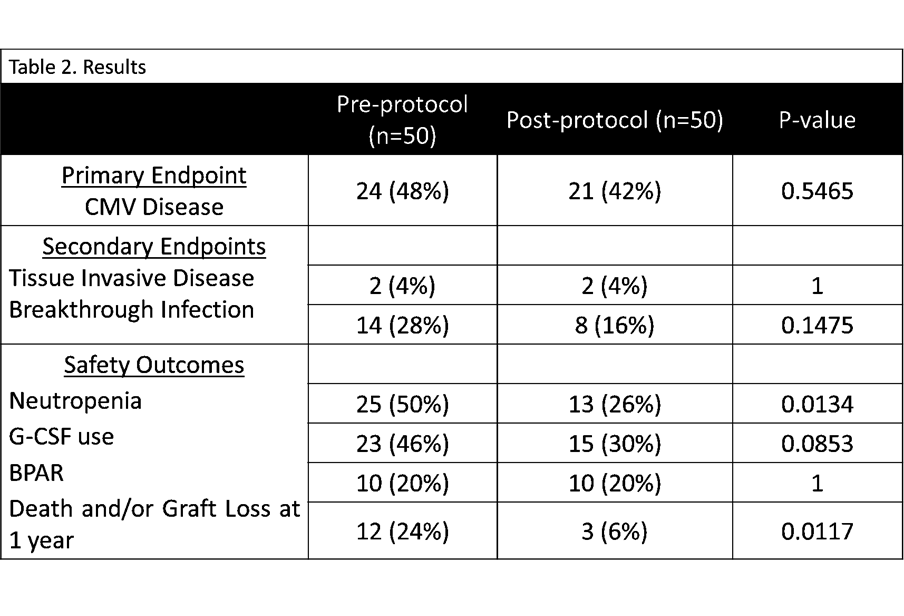
*Results: One hundred CMV D+/R- LT recipients were included. No statistical difference was found in the incidence of CMV disease at 1 year (p=0.5465), tissue invasive disease (p=1) or breakthrough CMV infections (p=0.1475). Only 1 patient in the post-protocol group developed resistance. With regard to safety outcomes, there was no difference in GCSF use or BPAR, however there was significantly more death and graft loss observed at 1 year in pre-protocol group versus the post-protocol group (p=0.0117). Neutropenia was also more common in the pre-protocol group.
*Conclusions: In a study of 100 high risk CMV LT patients, low-dose, delayed VGCV appears to be a safe and effective approach to managing CMV prophylaxis. There was no difference in incidence of CMV, resistance, breakthrough infection, or tissue invasive disease at 1 year. This approach had favorable outcomes, demonstrating less neutropenia, death and graft loss at 1 year. Delaying VGCV by up to 10 days has provided a safe and effective protocol for CMV prevention for LT recipients, while also providing a cost-containment strategy for the hospital.
To cite this abstract in AMA style:
Anders S, Freeman A, Hutchinson L, Kaszubski U, Janusek M, Hooter A, Raymond D, Nguyen C. Efficacy and Safety of Delayed, Low-Dose Valganciclovir in High-Risk Cytomegalovirus Liver Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/efficacy-and-safety-of-delayed-low-dose-valganciclovir-in-high-risk-cytomegalovirus-liver-transplant-recipients/. Accessed March 7, 2026.« Back to 2022 American Transplant Congress