Effect of Race and Antibody-Mediated Injury on Outcomes after Early Subclinical Inflammation
University of Alabama, Birmingham, AL
Meeting: 2019 American Transplant Congress
Abstract number: D99
Keywords: African-American, Antibodies, Graft survival, Protocol biopsy
Session Information
Session Name: Poster Session D: Kidney Acute Antibody Mediated Rejection
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: The impact of subclinical inflammation (SCI) on transplant outcomes has been variable, based on previous studies in homogeneous cohorts with relatively low immunologic risk. The purpose of this study was to define the incidence of SCI phenotypes and their impact on 3-year outcomes in a heterogeneous cohort with increased immunologic risk.
*Methods: We performed a cohort study of the universal surveillance biopsy program at our center. We included all kidney biopsies performed between May 2015 (the start of our surveillance biopsy program) and December 2017. The primary exposure was SCI, inclusive of subclinical T cell-mediated rejection (SC-TCMR), subclinical borderline TCMR (SC-B-TCMR), and subclinical antibody-mediated rejection (SC-ABMR), treated at the discretion of the attending clinician. The primary outcome was a composite endpoint of acute rejection or death-censored graft loss by 3 years. We examined the effect of SCI on the composite endpoint using Kaplan-Meier methods and adjusted for the effect of clinical covariates using Cox regression.
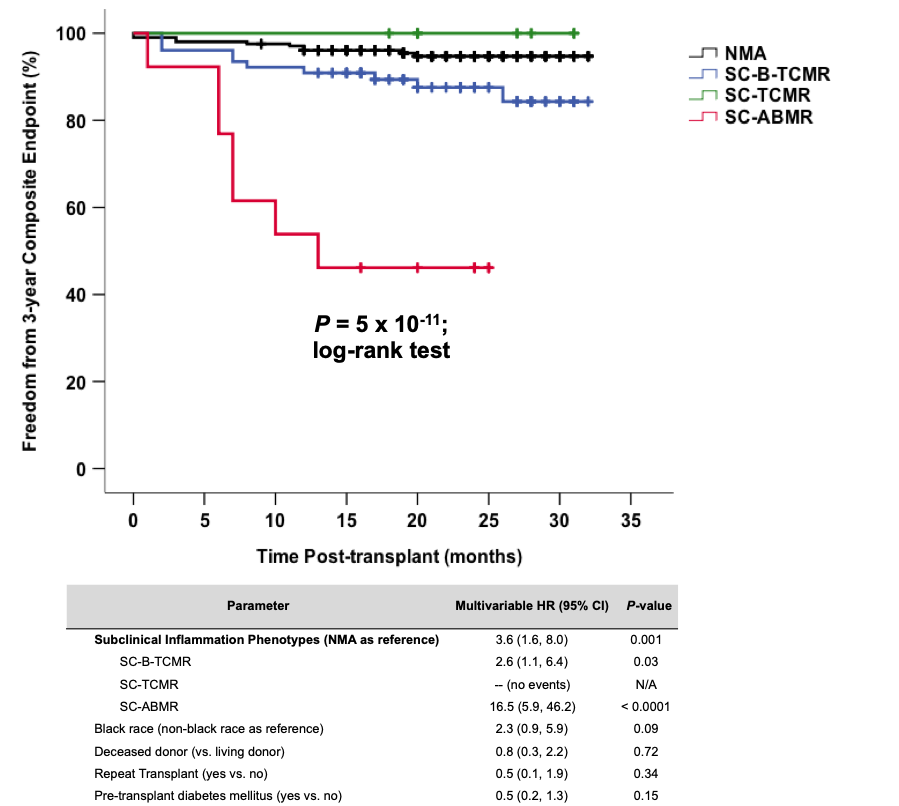
*Results: We reviewed 913 biopsies from 716 kidney recipients during the study period, of which 303 were identified as 6-month surveillance. Our surveillance cohort had high-risk features including 61% deceased donor type, 55% black race, 52% glomerular/diabetic cause of end-stage kidney disease, and 11% repeat transplants. We detected SCI in 98/303 (32%) biopsies, including SC-TCMR in 8 (3%), SC-B-TCMR in 77 (25%), and SC-ABMR in 13 (4%) recipients. Of these, 42 were treated with increased immunosuppression (mostly SC-TCMR and SC-ABMR) whereas 56 were managed expectantly (mostly SC-B-TCMR). Overall, the 3-year composite endpoint was met in 27 (9%) recipients but occurred significantly more often in those with SCI versus no major abnormalities (NMA) (17% vs. 5%; P=0.0004). The impact of SCI on the composite endpoint was most profound within black recipients [common odds ratio (95% CI) 4.0 (1.7, 9.1); P=0.001]. However, the SC-ABMR phenotype of SCI had the worst composite outcome even after adjusting for high-risk clinical features.
*Conclusions: We detected SCI in 32% of high-risk recipients at 6 months post-transplant, much higher than previously reported. Black race strongly influenced the overall impact of SCI, but the SC-ABMR phenotype was associated with the worst 3-year outcomes after SCI. This study highlights the need for targeted interventions for improving outcomes in those with high-risk SCI phenotypes.
To cite this abstract in AMA style:
Kasik EP, Seifert ME, Bernard M, Agarwal G, Fatima H, Gaston R, Julian B, Kew C, Kumar V, Mehta S, Ong S, Rosenblum F, Towns G, Mannon RB. Effect of Race and Antibody-Mediated Injury on Outcomes after Early Subclinical Inflammation [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/effect-of-race-and-antibody-mediated-injury-on-outcomes-after-early-subclinical-inflammation/. Accessed July 3, 2025.« Back to 2019 American Transplant Congress