Early Post-Transplant Immunosuppression (IS) Withdrawal – Final Outcomes of the ITN030ST AWISH Study.
1UPenn, Philadelphia
2UCSF, San Francisco
3U Michigan, Ann Arbor
4U Washington, Seattle
5Northwestern, Chicago
6Baylor, Dallas
7RhoFed, Chapel Hill
8NIAID, Bethesda.
Meeting: 2016 American Transplant Congress
Abstract number: 185
Keywords: Immunosuppression, Liver transplantation, Tolerance
Session Information
Session Name: Concurrent Session: Clinical Science: Tolerance: Clinical Studies
Session Type: Concurrent Session
Date: Monday, June 13, 2016
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:30pm-2:42pm
Presentation Time: 2:30pm-2:42pm
Location: Room 304
ITN030ST is a prospective randomized trial designed to determine the clinical benefit of early IS withdrawal in adult liver transplant (LT) recipients. Recipients did not receive induction therapy and were maintained on standard CNI IS.
275 participants were enrolled at LT across 7 US sites; 145 had non immune non-viral (NINV) causes and 130 had hepatitis C virus (HCV) as indication for LT. Subject disposition is shown in Figure 1: 199 (72%) achieved IS monotherapy early after LT (median 218[IQR 124-326]days). Subjects achieving stable monotherapy, acceptable liver function, and biopsy without rejection or fibrosis (n=95) were randomized to withdrawal (n=77) or maintenance (n=18) at a mean of 17±4.5 months post-LT. Major reasons disqualifying randomization were voluntary withdrawal (n=41), HCV activity (n=40), and protocol deviation (n=19).
Response to IS withdrawal was similar for HCV or NINV subjects. 52/77 (68%) achieved ≤50% of baseline dose. 10/77 (13%) were off IS ≥ 1 year: 9 remained IS free until study completion (24 months of follow-up) and 1 until retransplantation for HCV recurrence. Of 67 who failed withdrawal, 30 had biopsy-proven rejection and 37 had abnormal liver tests; all resolved with CNI therapy with or without antimetabolites. Only 13 (19%) subjects required corticosteroids.
The primary composite endpoint, evaluated 24 months after randomization, was comprised of death or graft loss; grade 4 malignancy or opportunistic infection; stage ≥3 fibrosis; or 25% decrease in GFR. The endpoint occurred in 4 of 13 (31%) and 12 of 66 (18%) evaluable subjects in the maintenance and withdrawal groups respectively.
Conclusion: IS minimization starting 12-24 months after LT was tolerated by the majority of recipients. Complete IS withdrawal was achieved in 13% of those qualified for the minimization protocol.
Figure 1. Participant Disposition 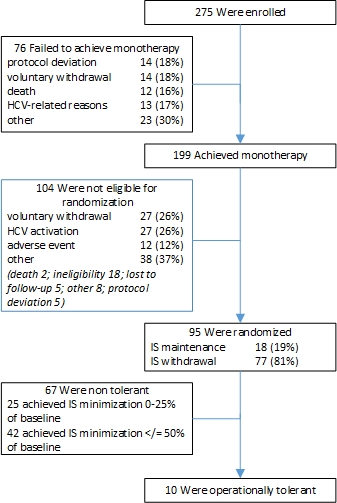
CITATION INFORMATION: Shaked A, Feng S, Punch J, Reyes J, Levitsky J, Klintmalm G, Kopetskie H, DesMarais M, Priore A, Bridges N, Sayre P. Early Post-Transplant Immunosuppression (IS) Withdrawal – Final Outcomes of the ITN030ST AWISH Study. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Shaked A, Feng S, Punch J, Reyes J, Levitsky J, Klintmalm G, Kopetskie H, DesMarais M, Priore A, Bridges N, Sayre P. Early Post-Transplant Immunosuppression (IS) Withdrawal – Final Outcomes of the ITN030ST AWISH Study. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/early-post-transplant-immunosuppression-is-withdrawal-final-outcomes-of-the-itn030st-awish-study/. Accessed March 9, 2026.« Back to 2016 American Transplant Congress
