Dosing Requirements Of Astagraf Xl In African American Kidney Transplant Recipients Converted From Twice-daily Tacrolimus (aaaktrs)
Medical University of South Carolina, Charleston, SC
Meeting: 2019 American Transplant Congress
Abstract number: A246
Keywords: African-American, Dosage, Gene expression, Kidney transplantation
Session Information
Session Name: Poster Session A: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
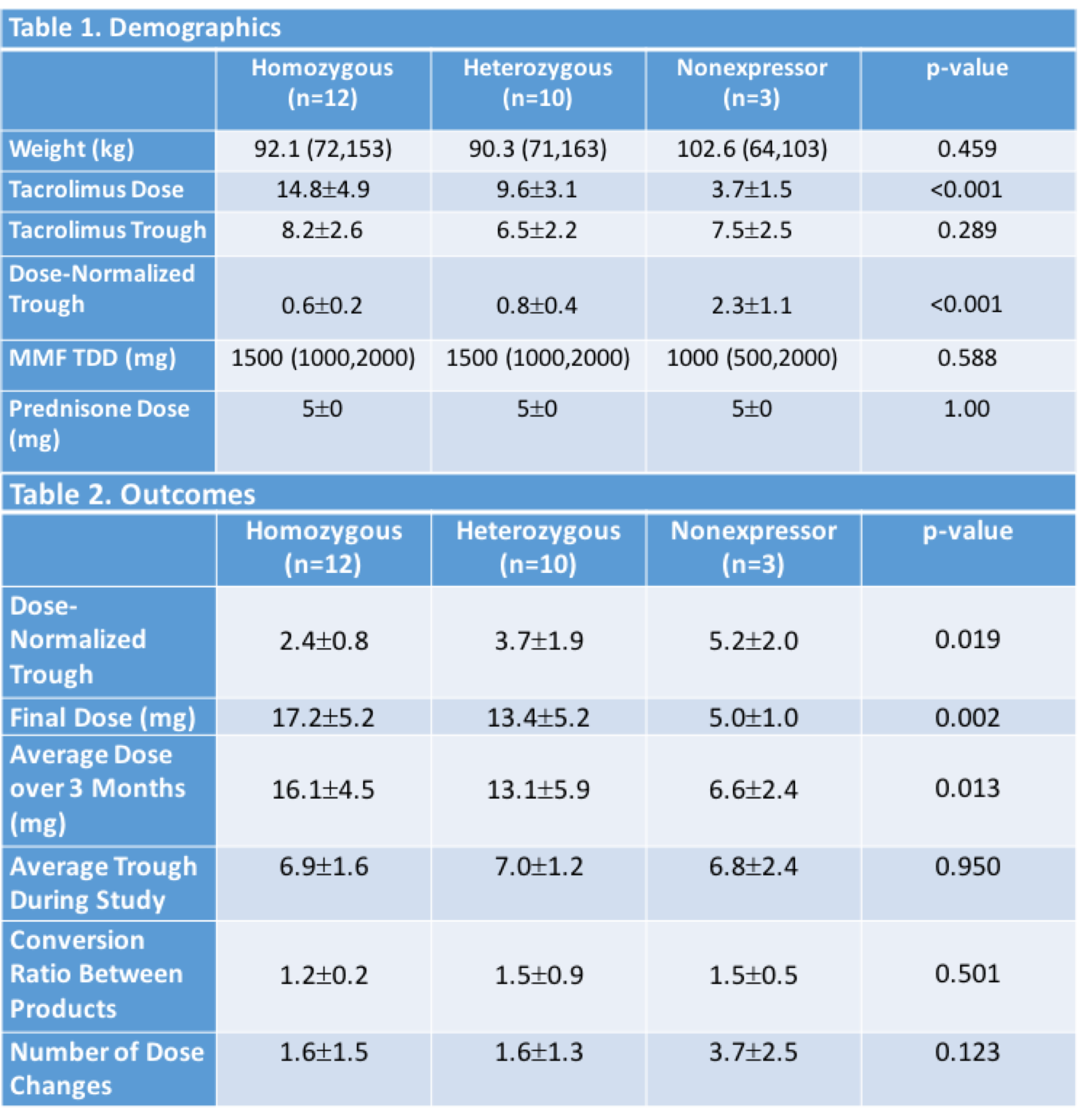
*Purpose: Pharmacokinetics from clinical studies demonstrated similar drug exposure (AUC) and correlation between trough (C0) and AUC between tacrolimus IR and Astagraf XL, which led to a recommendation for a 1:1 dose-conversion ratio. AA were underrepresented in these studies, casting doubt on the appropriate conversion ratio for this population. More recent studies have shown a decrease in FK exposure after conversion and increased dosing requirements in 25-50% of patients, which may be partially due to CYP3A5 alleles. De novo dosing of Astagraf XL has demonstrated that AA require 20-30% higher doses than Caucasians from 1-12 months after txp to achieve similar C0s. The purpose of this study was to compare the dose-normalized trough, total daily dose, and conversion ratio necessary to reach therapeutic goals after conversion from tacrolimus IR to Astagraf XL in AA KTx recipients.
*Methods: This was a single-center, prospective, open-label pilot study including conversion from tacrolimus IR to Astagraf XL in 25 AA KTx recipients with a 3 month comparison in dosing and C0 between CYP3A5 non-expressors (NE), heterozygous expressors (HE), and homozygous expressors (HO).
*Results: Of the 25 AA included, 3 were NE, 10 were HE, and 12 were HO. There were no differences in FK trough at baseline, but FK dose and dose-normalized C0 differed significantly (Table 1). After conversion to Astagraf XL, there was no difference in avg C0 throughout the study, but there remained significant differences between groups for avg dose and dose-normalized C0. Conversion ratios between tacrolimus IR and Astagraf XL were 1.2 for HO and 1.5 for HE and NE, respectively (Table 2).
*Conclusions: Our study confirmed a need for an non-1:1 conversion ratio between tacrolimus IR and Astagraf XL in AA KTx, which differed based on CYP3A5 expression. Further studies are warranted to clarify the appropriate conversion ratio and role of CYP3A5 genotype in dose decisions.
To cite this abstract in AMA style:
Fleming JN, Posadas-Salas MA, Taber DJ. Dosing Requirements Of Astagraf Xl In African American Kidney Transplant Recipients Converted From Twice-daily Tacrolimus (aaaktrs) [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/dosing-requirements-of-astagraf-xl-in-african-american-kidney-transplant-recipients-converted-from-twice-daily-tacrolimus-aaaktrs/. Accessed March 7, 2026.« Back to 2019 American Transplant Congress