Donor-Derived Monocytes Migrate to the Contralateral Lung and Cause Neutrophilia Following Single Lung Transplant.
Northwestern University, Chicago, IL.
Meeting: 2016 American Transplant Congress
Abstract number: 203
Keywords: Lung transplantation, Mononuclear leukocytes, Neutrophils
Session Information
Session Name: Concurrent Session: Innate Immunity in Rejection and Tolerance: Animal Models
Session Type: Concurrent Session
Date: Monday, June 13, 2016
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:18pm-3:30pm
Presentation Time: 3:18pm-3:30pm
Location: Room 313
Purpose: Deterioration of the native lung from lung injury occurs in the majority of patients following single lung transplant, but its pathogenesis remains unknown. Here, we demonstrate that pulmonary intravascular CX3CR1highLy6Clow monocytes (Ly6ClowM) in the donor lungs migrate to the native lung and lead to infiltration of neutrophils, the key mediators of lung injury.
Methods: Allogeneic single lung transplant was performed utilizing wild-type, CX3CR1gfp/+, and CX3CR1 knockout (KO) mice. Donor lungs were flushed using standard preservatives. Intravenous clodronate liposomes (IV Clo-lip) were used to deplete intravascular monocytes. Lung myeloid populations, including neutrophils and Ly6ClowM, were identified by flow cytometry. Data are presented as mean ± SEM. Student's t-test was used to compare means. Experimental groups consisted of n=2-8.
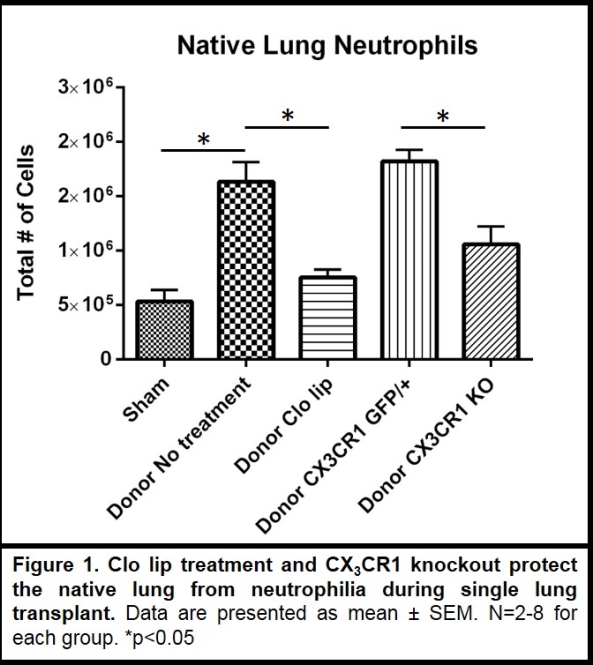
Results: Donor lungs revealed 2-5% of myeloid cells to be intravascular Ly6ClowM. At 24 hours following allogeneic single lung transplant, native lungs demonstrated a 3-fold increase in neutrophils compared to sham thoracotomy (1.6±0.2×106 v. 0.5±0.1×106, p=0.02). Using CD45.1 donors and CD45.2 recipients, 8% of the Ly6ClowM in the native lung were of donor origin. IV Clo-lip depleted Ly6ClowM in donor lungs (<0.5%). Allogeneic recipients of Ly6ClowM-depleted donor lungs demonstrated no donor-derived Ly6ClowM in the native lung and markedly decreased neutrophilia (0.8±0.1×106 vs. 1.6±0.2×106, p<0.01). Similarly, CX3CR1 KO donor lungs showed decreased Ly6ClowM and recipients of CX3CR1 KO lungs revealed suppressed native lung neutrophilia compared to recipients of CX3CR1gfp/+ lungs that have preserved Ly6ClowM (1.1±0.2 v. 1.8±0.1×106, p=0.04).
Conclusion: Donor-derived pulmonary intravascular Ly6ClowM migrate to the native lung following single lung transplant and induce neutrophilia. Depletion of these monocytes in the donor lung protects the native lung from neutrophilia.
CITATION INFORMATION: Chiu S, Zheng Z, Sun H, DeCamp, Jr M, Budinger G, Perlman H, Misharin A, Bharat A. Donor-Derived Monocytes Migrate to the Contralateral Lung and Cause Neutrophilia Following Single Lung Transplant. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Chiu S, Zheng Z, Sun H, DeCamp M, Budinger G, Perlman H, Misharin A, Bharat A. Donor-Derived Monocytes Migrate to the Contralateral Lung and Cause Neutrophilia Following Single Lung Transplant. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/donor-derived-monocytes-migrate-to-the-contralateral-lung-and-cause-neutrophilia-following-single-lung-transplant/. Accessed March 2, 2026.« Back to 2016 American Transplant Congress
