Donor-Derived Cell-Free DNA is Not Influenced by Steroid Dosing in Heart Transplant Recipients
1Stanford University, Stanford, CA, 2Ochsner Medical Center, New Orleans, LA, 3West Virginia University, Morgantown, WV, 4Pennsylvania State University, Hershey, PA
Meeting: 2020 American Transplant Congress
Abstract number: D-252
Keywords: Glucocortocoids, Heart, Non-invasive diagnosis
Session Information
Session Name: Poster Session D: Biomarkers, Immune Assessment and Clinical Outcomes
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Gene expression profiling (GEP) was recognized as an alternative to biopsy for rejection surveillance in the 2010 International society for Heart and Lung Transplantation guidelines for the care of heart transplant recipients. However, GEP (AlloMap®) is only recommended for use > 55 days after heart transplant (HTx). Furthermore, the GEP scores can be falsely low when prednisone doses are 20mg or greater. In contrast, donor derived cell-free DNA (dd-cfDNA, AlloSure®) can be used as soon as two weeks post-HTx and in the setting of higher steroid doses, although the impact of steroid dosing on dd-cfDNA has not been systematically examined in routine clinical use.
*Methods: An assessment of the dd-cfDNA from HTx recipients in the DOAR and SHORE registries, regardless of time post-HTx, was compared among prednisone doses categorized: as 0-5, 5-10, 10-15, 15-20, and >20 mg/day. Testing of dd-cfDNA < 55 days (EARLY) post-HTx was compared to between 55 and 90 days post-HTx (LATE) in samples from SHORE. Differences in score distributions between groups were assessed by the Kolmogorov-Smirnov test.
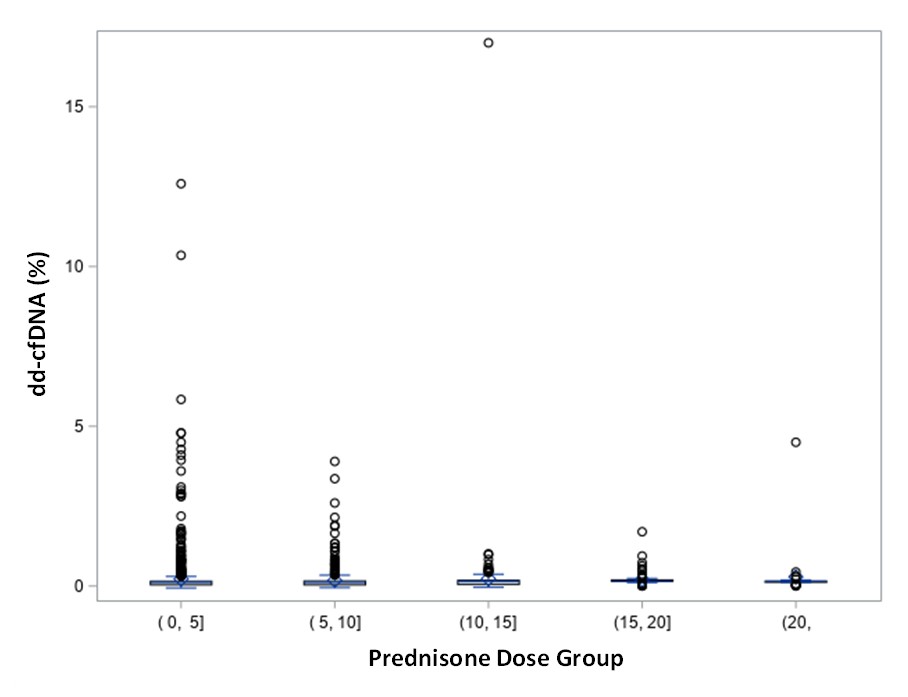
*Results: 816 adult patients from D-OAR and SHORE were included in the analysis of steroid dose impact. The 816 patients had 1970 samples; 1113 (56.5%) samples from patients on 0-5mg of prednisone at the time of sampling, 542 (27.5%) on 5-10mg, 203 (10.3%) on 10-15mg, 82 (4.2%) on 15-20mg, and 30 (1.5%) on >20mg. Across steroid doses, the median dd-cfDNA result for 0-5, 5-10, 10-15, 15-20, and >20mg was 0.12%, 0.12%, 0.15%, 0.15%, and 0.15%, respectively, p=0.0813 (Figure). A time post-HTx analysis included 94 patients from SHORE with 17 visits for the EARLY group and 106 for the LATE group. The median dd-cfDNA for EARLY was 0.15% (IQR: [0.15%, 0.19%]) and for LATE was 0.15% (IQR: [0.15%, 0.15%]), with no significant differences between EARLY and LATE, p=0.7754. There was one ACR in the EARLY group, and none in the LATE group.
*Conclusions: Neither steroid dose nor testing before or after 55 days impacted the dd-cfDNA results for the low risk patients followed in the DOAR/SHORE database. The use of dd-cfDNA should be considered as a non-invasive biomarker for rejection surveillance regardless of steroid dosing and as soon as 14 days after HTx.
To cite this abstract in AMA style:
Teuteberg J, Krim S, Shullo M, Jimenez S, Eisen H. Donor-Derived Cell-Free DNA is Not Influenced by Steroid Dosing in Heart Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/donor-derived-cell-free-dna-is-not-influenced-by-steroid-dosing-in-heart-transplant-recipients/. Accessed February 26, 2026.« Back to 2020 American Transplant Congress