Donor-Derived Cell-Free DNA Correlates with Antibody-Mediated Rejection in Kidney Allografts.
1University of Pennsylvania, Philadelphia, PA
2University of Maryland, Baltimore, MD
3Cleveland Clinic, Cleveland, OH
4CareDx, Brisbane, CA
5Washington University, St. Louis, MO
Meeting: 2017 American Transplant Congress
Abstract number: 397
Keywords: Monitoring, Multicenter studies, Non-invasive diagnosis, Rejection
Session Information
Session Name: Concurrent Session: Diagnosis of Antibody Mediated Rejection in Kidney Transplant Recipients
Session Type: Concurrent Session
Date: Tuesday, May 2, 2017
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:30pm-2:42pm
Presentation Time: 2:30pm-2:42pm
Location: E354a
Purpose: Donor-derived cell-free DNA (dd-cfDNA) is a noninvasive test of allograft injury that may enable more frequent, quantitative, and safer assessment of allograft rejection and injury status. Analysis of data from a large multi-center study determined how well dd-cfDNA correlates with T cell-mediated rejection or antibody-mediated rejection (ABMR).
Methods: We analyzed blood specimens from all kidney recipients in the 14-center study that had a clinically-indicated biopsy (n=102). Plasma dd-cfDNA was quantified in a CLIA laboratory using a clinical-grade targeted next generation sequencing method that does not require separate genotyping of the donor or recipient. The fraction of total cf-DNA originating from the graft was compared to histopathology results as defined by established Banff criteria.
Results: The dd-cfDNA level discriminated biopsy specimens with antibody-mediated rejection from those with no ABMR (p<0.001), with a receiver-operating-characteristic (ROC) area under the curve (AUC) of 0.87 (95% confidence interval (CI) 0.75-0.97). The AUC for discriminating any rejection (TCMR Type IA or greater or ABMR) from samples without rejection was 0.74 (95% CI 0.61-0.86). The positive and negative predictive values for ABMR at a cutoff of 1.0% dd-cfDNA were 44% and 96%, respectively. The positive and negative predictive values for any rejection at a cutoff of 1.0% were 61% and 84%, respectively. Median dd-cfDNA was 2.9% (antibody-mediated rejection), 1.2% (TCMR, Types ≥ IB), 0.2% (TCMR rejection Type IA), and 0.3% in controls (p<0.001 for ABMR vs controls; p=0.05 for TCMR Types ≥ IB vs controls). The correlation of dd-cf-DNA to Banff elemental lesions shown in the figure.
Conclusions: dd-cfDNA may be used to assess allograft rejection and injury; levels ≥1% indicate a high probability of antibody mediated rejection. dd-cfDNA levels below 1% reflect absence of active antibody rejection.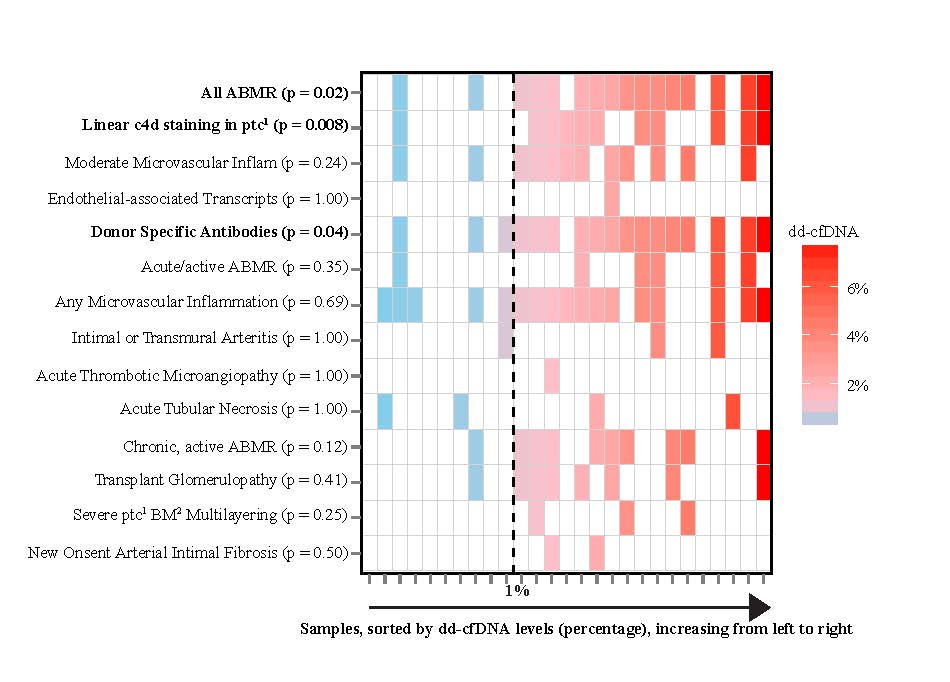
CITATION INFORMATION: Bloom R, Bromberg J, Poggio E, Hiller D, Woodward R, Sninsky J, Yee J, Brennan D. Donor-Derived Cell-Free DNA Correlates with Antibody-Mediated Rejection in Kidney Allografts. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Bloom R, Bromberg J, Poggio E, Hiller D, Woodward R, Sninsky J, Yee J, Brennan D. Donor-Derived Cell-Free DNA Correlates with Antibody-Mediated Rejection in Kidney Allografts. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/donor-derived-cell-free-dna-correlates-with-antibody-mediated-rejection-in-kidney-allografts/. Accessed March 4, 2026.« Back to 2017 American Transplant Congress
