Divergent Molecular Pathways Underlie the Evolution of Allograft Fibrosis in Two Mouse Models of Chronic Lung Allograft Dysfunction
Latner Thoracic Surgery Research Laboratories, Toronto Lung Transplant Program / University Health Network, Toronto, ON, Canada
Meeting: 2020 American Transplant Congress
Abstract number: 82
Keywords: Fibrosis, Lung transplantation
Session Information
Session Name: From Bench to Community to Bedside in Lung Transplantation
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:15pm-3:27pm
Presentation Time: 3:15pm-3:27pm
Location: Virtual
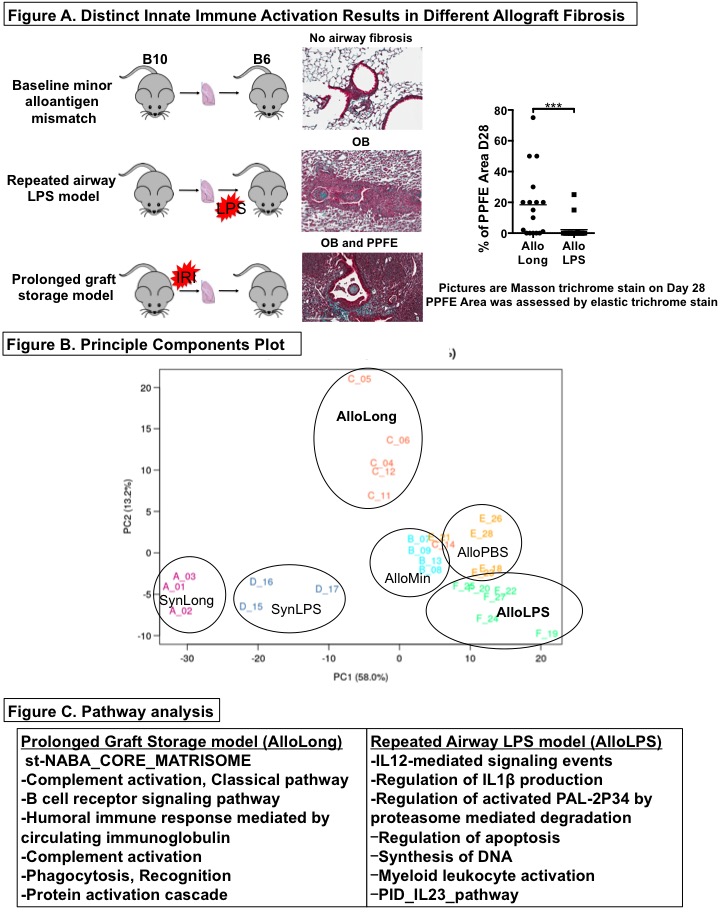
*Purpose: Chronic lung allograft dysfunction (CLAD) after lung transplantation (LTx) is potentiated by activation of the innate immune system and occurs in two patterns: diffuse pleuroparenchymal fibroelastosis (PPFE) and an airway-centered pathology, obliterative bronchiolitis (OB). Using a minor alloantigen-mismatched (C57BL/10 to C57BL/6) mouse LTx model, we have developed a model of PPFE-predominant fibrosis driven by ischemia-reperfusion injury (IRI) and a model of OB-predominant fibrosis driven by repeated airway instillation of lipopolysaccharide (LPS) (Figure A). We tested the hypothesis that the immune, inflammatory, and tissue remodelling pathways leading to these two phenotypes would differ and might reveal novel therapeutic targets.
*Methods: At days 14, RNA was extracted from mouse lung grafts and subjected to mRNA sequencing. To discern the contributions of the distinct innate stimuli (LPS and IRI) and the presence or absence of alloantigens (Syn- or Allografts), different groups of grafts (n=3-6 per group at each time point) were included: allografts with prolonged storage to induce IRI (AlloLong), allografts with minimal storage (AlloMin), syngrafts with prolonged storage (SynLong), allografts exposed to LPS (AlloLPS), allografts exposed to PBS (AlloPBS), and syngrafts exposed to LPS (SynLPS). RNAseq DESeq2 was used to perform differential discovery on the data, with the primary comparison being AlloLong versus AlloLPS. Pathway analysis was performed using the GO database.
*Results: The groups separated distinctly along two principal components in multidimensional space (Figure B). Analysis of differential gene expression indicated that AlloLPS grafts were enriched for neutrophil, monocyte and T cell signaling-related transcripts while AlloLong grafts were enriched for extracellular matrix remodelling, B cell and humoral immunity-related transcripts (Figure C). The observation of B cell transcripts in AlloLong grafts is consistent with our recent finding that fibrosis in this model is B cell dependent, and that the grafts contain tertiary lymphoid organs and extensive fibrosis resembling PPFE. AlloLPS grafts exhibit increased neutrophils and Th17 cells by flow cytometry and airway-centered OB-like lesions histologically.
*Conclusions: Our data suggest that the different patterns of CLAD pathology may be driven by distinct immune and tissue remodelling pathways.
To cite this abstract in AMA style:
Watanabe T, Allen J, Boonstra K, Guan Z, Bei K, Keshavjee S, Yeung J, Martinu T, Juvet S. Divergent Molecular Pathways Underlie the Evolution of Allograft Fibrosis in Two Mouse Models of Chronic Lung Allograft Dysfunction [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/divergent-molecular-pathways-underlie-the-evolution-of-allograft-fibrosis-in-two-mouse-models-of-chronic-lung-allograft-dysfunction/. Accessed February 19, 2026.« Back to 2020 American Transplant Congress