Development of a Novel Assay System for Ex Vivo T-Cell Mediated Rejection Responses in Intestinal Grafts
S. Okano,1,2 K. Abu-Elmagd,2 D. Kish,1 K. Keslar,1 M. Fujiki,2 G. Costa,2 C. Miller,2 W. Baldwin,1 R. Fairchild,1 K. Hashimoto.2
1Immunology, Lerner Research Institute, Cleveland Clinic, Cleveland, OH
2General Surgery, Digestive Disease & Surgery Institute, Cleveland Clinic, Cleveland, OH.
Meeting: 2018 American Transplant Congress
Abstract number: 86
Keywords: Graft-infiltrating lymphocytes, Intestinal transplantation, Rejection, T cells
Session Information
Session Name: Concurrent Session: Small Bowel: All Topics
Session Type: Concurrent Session
Date: Sunday, June 3, 2018
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:18pm-3:30pm
Presentation Time: 3:18pm-3:30pm
Location: Room 210
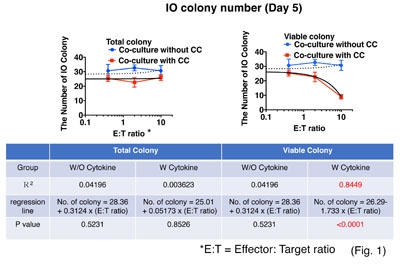
In intestinal transplantation, the intestinal allograft (IA) presents a formidable challenge for control of alloimmune responses due to constitutive MHC class II expression by intestinal epithelial cells (IE), abundant mobile hematopoietic cells (mHC), and continuous exposure to gut microbiota. Mechanisms and regulation of donor-specific T cell-mediated allograft rejection responses (DSTR) within IA are unclear due to the lack of a clinically relevant model. We developed a novel ex vivo assay system for DSTR using 3D intestinal organoid (IO) culture. Donor-specific CD4 and CD8 T cell lines (DST) were derived by co-culturing recipient peripheral blood mononuclear cells with irradiated donor-derived B cell lines (BL) and specificity confirmed by cytolysis of donor not recipient BL. Donor IOs from pre-transplant IA expressed both HLA-class I and II and were co-cultured with DST. Numbers of viable IOs were counted; a “viable organoid” defined as a translucent organoid with sharp border and without DST infiltration. The Caspase-3/7 activity of IOs were also measured. When donor IO were co-cultured with DST without extracellular matrix (ECM) for 2-16 hours, caspase-3/7 activation increased as a function of E:T after 4- and 16-hours. When the IOs were co-cultured with DST on ECM for 5 days with or without a cytokine cocktail (CC; IL-2, IL7, and IL-15), IO numbers in medium with CC decreased in an E:T-dependent manner (R2 =0.8449, p < 0.0001), but not those without CC (R2 = 0.04196, p = 0.5231) (Fig. 1). Addition of myeloid-derived suppressor cells (MDSC) regulated DST inhibition of donor IO formation. These results suggest that: i) DST directly induces Caspase 3/7-dependent apoptosis of IE; ii) T-cell growth factors are needed to sustain DSTR to IOs embedded in the matrix; and, iii) MDSCs suppress the DSTR. Our novel ex vivo DSTR assay system will be a useful tool for analysis of immunoregulation among IE, mHC, and DST within IA.
CITATION INFORMATION: Okano S., Abu-Elmagd K., Kish D., Keslar K., Fujiki M., Costa G., Miller C., Baldwin W., Fairchild R., Hashimoto K. Development of a Novel Assay System for Ex Vivo T-Cell Mediated Rejection Responses in Intestinal Grafts Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Okano S, Abu-Elmagd K, Kish D, Keslar K, Fujiki M, Costa G, Miller C, Baldwin W, Fairchild R, Hashimoto K. Development of a Novel Assay System for Ex Vivo T-Cell Mediated Rejection Responses in Intestinal Grafts [abstract]. https://atcmeetingabstracts.com/abstract/development-of-a-novel-assay-system-for-ex-vivo-t-cell-mediated-rejection-responses-in-intestinal-grafts/. Accessed March 7, 2026.« Back to 2018 American Transplant Congress