Development and Validation of a Medication Safety Dashboard in Veteran Transplant Recipients: Preliminary Results of a Multicenter Cluster-Randomized Controlled Clinical Trial
D. Taber1, R. Felkner2, K. Rife3, D. Cooney3, S. Laforest3, E. Santa4, N. Super5, C. Hall6, T. Goddard1, R. Alexander1
1MUSC, Charleston, SC, 2Madison VA, Madison, WI, 3Cleveland VA, Cleveland, OH, 4Atlanta VA, Atlanta, GA, 5Chicago VA, Chicago, IL, 6Charleston VA, Charleston, SC
Meeting: 2022 American Transplant Congress
Abstract number: 150
Keywords: Drug interaction, HMG-CoA reductase inhibitor, Metabolic disease, Rejection
Topic: Clinical Science » Pharmacy » 30 - Non-Organ Specific: Clinical Pharmacy/Transplant Pharmacotherapy
Session Information
Session Name: Non-Organ Specific: Clinical Pharmacy/Transplant Pharmacotherapy
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:40pm-5:50pm
Presentation Time: 5:40pm-5:50pm
Location: Hynes Room 312
*Purpose: In organ transplant, med errors, adverse drug events, and nonadherence lead to increased healthcare utilization and graft loss. Veterans with transplants are a high-risk population.
*Methods: A med safety dashboard was created to identify potential issues that included missing pertinent labs, concerning trends in labs, drug-drug interactions, immunosuppressant non-adherence (refill gaps, expired meds), and transitions in care. This system was tested through a 24-month, prospective, cluster-randomized controlled multicenter study. Pharmacists at 5 intervention sites used the dashboard to identify and address potential med safety issues, which was compared with usual care provided at 5 control sites. Interim findings regarding dashboard functionality and interventions are reported here.
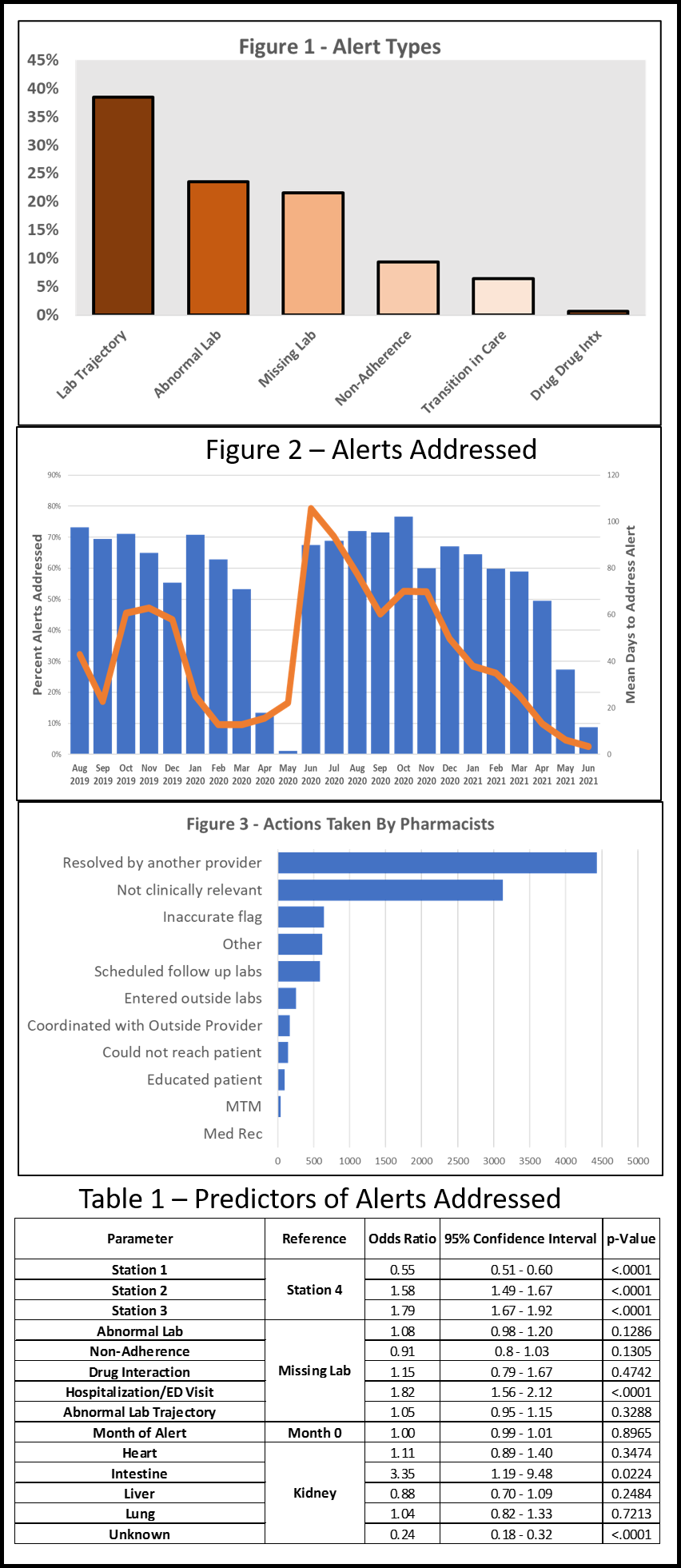
*Results: The study opened Mar 2019 and closed Jun 2021, with a COVID-19 induced hiatus (Apr to Jun 2020). As of the last interim analysis (18m follow-up), there were 1,928 patients enrolled across the 10 sites (1,181 intervention vs 815 control). Mean age was 65 years, 95% male, and 27% Black. Mortality was 9.3%, with no difference between arms (intervention 9.5% vs control 9.0%). ED visits (intervention 38.4% vs control 45.6%) and hospitalizations (intervention 25.6% vs control 37.6%) were higher in the control arm. The dashboard produced a total of 18,132 alerts from the 5 intervention sites; a rate of 1-2 per pt-month. Lab-based issues were most common (Figure 1), followed by non-adherence and transitions in care; 70% of alerts were addressed (Figure 2 blue bars) in about 40 days (Figure 2 orange line). Actions taken by pharmacists are displayed in Figure 3, which were often already addressed or not clinically relevant. Adjustments made to med regimens based on dashboard alerts were uncommon. Multivariable modeling demonstrated location site, type of alert, and transplant type were predictors of alerts being addressed (Table 1).
*Conclusions: This multicenter cluster-randomized controlled trial demonstrates that a med safety dashboard is feasibly deployable across the VA healthcare system, creating valid alerts; although most alerts were already addressed by other providers or deemed not to be clinically actionable. Future dashboard refinements should focus on reducing non-actionable alerts and addressing workload barriers to timely review.
To cite this abstract in AMA style:
Taber D, Felkner R, Rife K, Cooney D, Laforest S, Santa E, Super N, Hall C, Goddard T, Alexander R. Development and Validation of a Medication Safety Dashboard in Veteran Transplant Recipients: Preliminary Results of a Multicenter Cluster-Randomized Controlled Clinical Trial [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/development-and-validation-of-a-medication-safety-dashboard-in-veteran-transplant-recipients-preliminary-results-of-a-multicenter-cluster-randomized-controlled-clinical-trial/. Accessed February 23, 2026.« Back to 2022 American Transplant Congress