Detection of Early Graft Injury After Liver Transplantation with Blood-Bile Ratio of Tacrolimus: The TUBE Trial
1General Surgery and Liver Transplant Unit, Fondazione IRCSS - Policlinico "A. Gemelli", Roma, Italy, 2Transplant Hepatology Unit, Fondazione IRCSS - Policlinico "A. Gemelli", Roma, Italy
Meeting: 2022 American Transplant Congress
Abstract number: 1588
Keywords: FK506, Liver transplantation, Outcome, Rejection
Topic: Basic Science » Basic Clinical Science » 17 - Biomarkers: Clinical Outcomes
Session Information
Session Name: Biomarkers: Clinical Outcomes
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: The aim of the“Tacrolimus in Bile”(TUBE)Trial is the creation of a laboratory parameter for the early detection of hepatic injury after liver transplant.
*Methods: TUBE is a single-blind prospective monocentric trial,in which liver recipients who had Kehr’s tube inserted into the biliary tract were enrolled.In the first 10 postoperative days (POD),a bile sample was collected together with a blood sample.The blood and biliary values of Tacrolimus were correlated to create the”blood-bile ratio of Tacrolimus”(BBRT).The primary outcome was the assessment of the predictive ability of BBRT in the evaluation of liver rejection injury,diagnosed through laboratory or pathological analysis.The relationship between BBRT and liver injury was examined through a Wilcoxon-Mann-Whitney test.A ROC curve was developed to estimate the BBRT threshold with the best sensitivity/specificity ratio.
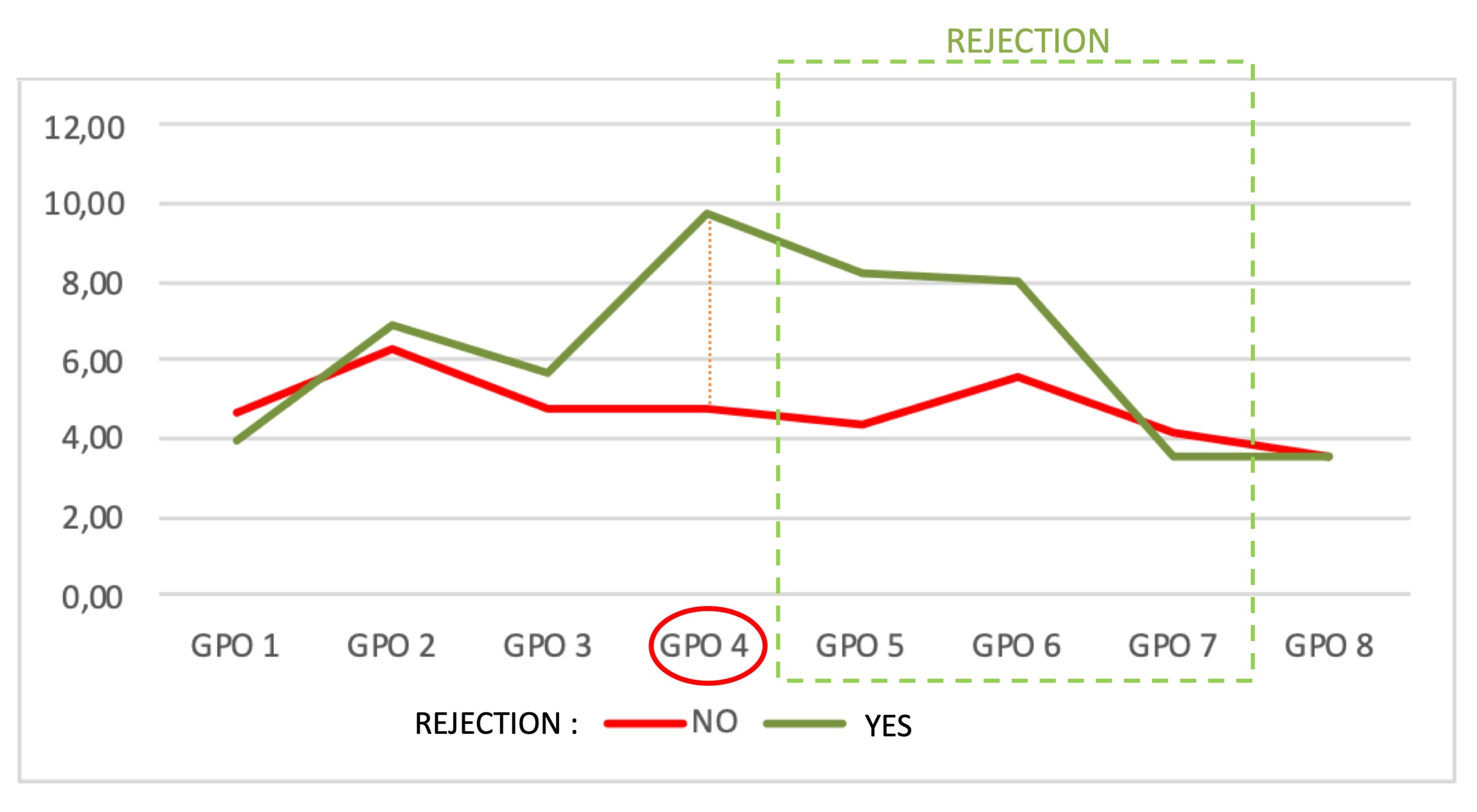
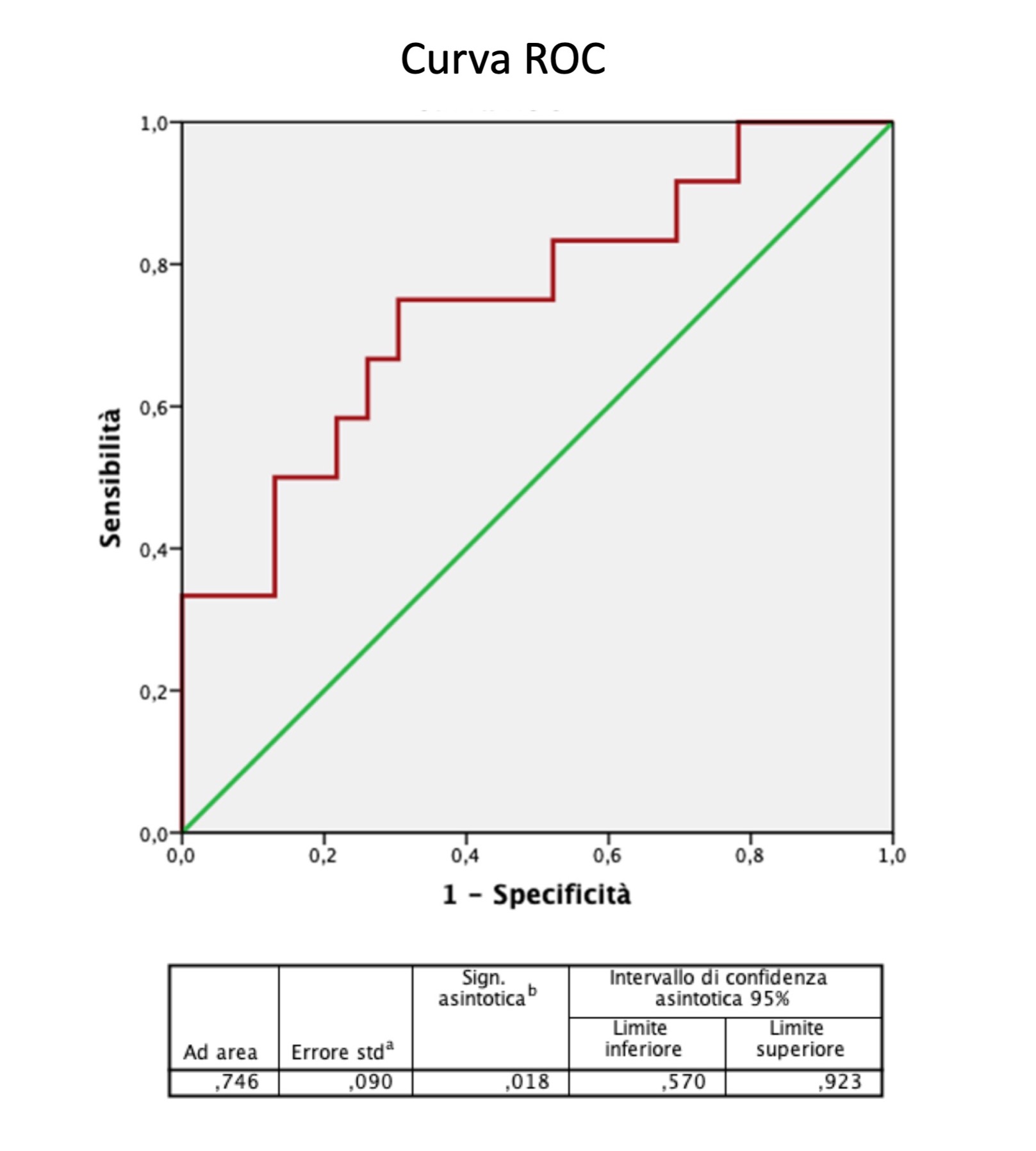
*Results: Among the 68 liver transplants performed in our institution, 35 patients enrolled.Twelve patients(34%) presented with acute rejection liver injury,diagnosed by standard methods between the 5thand 7thPOD.Transaminases,total bilirubin and blood tacrolimus levels did not differ significantly in the two study patient groups,unlike eosinophils within the rejection period.The mean BBRT value presented a significant difference between the two study groups already in the 4thPOD(p=0.026)(Figure 1).The ROC curve confirmed the statistical significance of BBRT(p=0.018).The sensitivity and specificity achieved with a BBRT cut-off value of 4.1 was 75% and 74%,respectively.
*Conclusions: The TUBE trial is the first study evaluating the relationship between blood and biliary concentrations of a marker with hepatic excretion in liver transplant patients.This research field can be deepened by considering other aspects,such as liver enzyme polymorphism or blood flow alterations to the graft.To date,BBRT can be considered an additional laboratory marker for detecting liver rejection damage after liver transplantation.
To cite this abstract in AMA style:
Pascale MM, Bianco G, Ferri L, Giovinazzo F, Liguori A, Marrone G, Grieco A, Agnes S. Detection of Early Graft Injury After Liver Transplantation with Blood-Bile Ratio of Tacrolimus: The TUBE Trial [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/detection-of-early-graft-injury-after-liver-transplantation-with-blood-bile-ratio-of-tacrolimus-the-tube-trial/. Accessed March 2, 2026.« Back to 2022 American Transplant Congress