Desensitization Protocol to Prevent Severe Oliguric Antibody-Mediated Rejection
1Tokyo Womens, Tokyo 162-8666, Japan, 2Tokyo Women's Medical University, Tokyo, Japan
Meeting: 2022 American Transplant Congress
Abstract number: 1024
Keywords: Alloantibodies, CD20, IVIG, Kidney
Topic: Clinical Science » Kidney » 36 - Kidney Immunosuppression: Desensitization
Session Information
Session Name: Kidney Immunosuppression: Desensitization
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
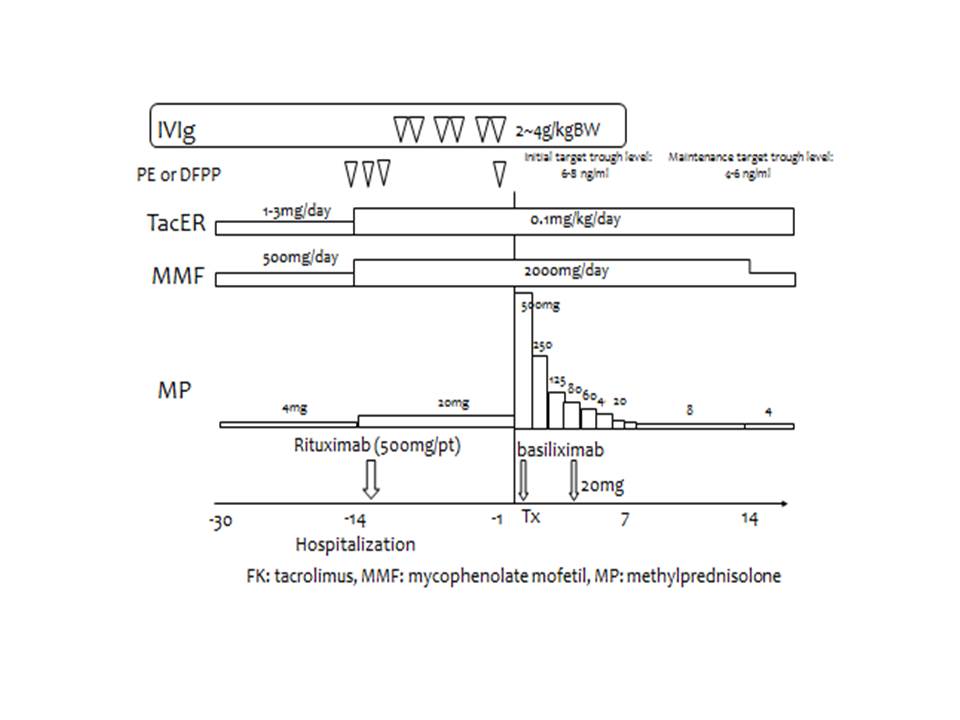
*Purpose: Antibody-mediated rejection in a case with a positive crossmatch test can be severe and result in sudden onset of oliguria and sometimes, anuria, leading to graft loss. Some researchers have reported rescue therapies, including B cell-targeting treatments, for oliguria or anuria. In an attempt to prevent oliguria/anuria in the immediate postoperative period, we adopted a preoperative desensitization protocol involving the use of high-dose IVIg (H-IVIg)/plasmapheresis (PP) and the anti-CD20 antibody, rituximab (Rit), in 41 recipients with positive crossmatch test results prior to renal transplantation.
*Methods: We adopted this desensitization protocol in a total of 41 recipients with positive results of crossmatch tests between 2011 and 2020. We retrospectively examined the clinical courses of these recipients after kidney transplantation, paying special attention to the renal graft function, urine volume and changes in the titers of donor-specific antibodies following the transplantation. The primary endpoint of this single-center study was the urine output after kidney transplantation in sensitized recipients who received our latest desensitization protocol, including use of H-IVIg, at our institution.
*Results: Four grafts were lost during an average of 4.5 years’ follow-up: one each due to suicide and chronic antibody-mediated rejection, and the remaining two due to cardiovascular events. The average graft function was excellent, with a serum creatinine level of 1.3±0.4 mg/dL. Sufficient urine output, with no oliguria or anuria, was achieved postoperatively in 40 out of the 41 patients. However, among the 34 patients who underwent graft biopsies, the graft biopsies revealed acute antibody-mediated rejection in 21 patients (21/34, 62%), acute T cell-mediated rejection in 14 patients (14/34, 41%), and chronic antibody-mediated rejection in 10 patients (10/34, 30%).
*Conclusions: Our latest desensitization protocol, including use of H-IVIg/PP and Rit, has proven effective for preventing postoperative oliguria/anuria in kidney transplant recipients. The H-IVIg treatment included in our desensitization protocol is safe and effective for achieving successful transplantation, and allows the avoidance of more aggressive B cell-targeting treatments, such as like C5 inhibitors and/or proteosome inhibitors, for preventing postoperative oliguria/anuria.
To cite this abstract in AMA style:
Ishida H, Unagami K, Kamzawa T, Omoto K, Shimizu H, Tanabe K. Desensitization Protocol to Prevent Severe Oliguric Antibody-Mediated Rejection [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/desensitization-protocol-to-prevent-severe-oliguric-antibody-mediated-rejection/. Accessed March 5, 2026.« Back to 2022 American Transplant Congress