Desensitization Protocol Including Concomitant Bortezomib and Rituximab Did Not Reduce Calculated PRA Nor Mean MFI Values Among Waitlisted Patients for Deceased Donor Kidney Transplantation
1Transplantation Center, Seoul National University Hospital, Seoul, Korea
2Department of Laboratory Medicine, Seoul National University Hospital, Seoul, Korea
3Department of Surgery, Konkuk University, Seoul, Korea.
Meeting: 2015 American Transplant Congress
Abstract number: C91
Keywords: Highly-sensitized, HLA antibodies, Kidney transplantation
Session Information
Session Name: Poster Session C: Kidney Immunosuppression: Desensitization
Session Type: Poster Session
Date: Monday, May 4, 2015
Session Time: 5:30pm-6:30pm
 Presentation Time: 5:30pm-6:30pm
Presentation Time: 5:30pm-6:30pm
Location: Exhibit Hall E
Highly sensitized (HS) patients (pts) seldom have chance to receive deceased donor kidney transplant (DDKT). A desensitization (DES) program including bortezomib was employed to facilitate DDKT in HS waitlisted pts.
We prospectively enrolled 19 pts. DES protocol consisted of IVIG (2g/kg, D0 and 30), rituximab (375 mg/m2, D1), and bortezomib (1.3mg/m2 on D14, 17, 21 and 24). Anti-HLA antibodies(Abs) were evaluated at baseline and at months 1, 2 and 5 after DES using single antigen Luminex assay. We compared transplant conversion rate and PRAs between DES and non-DES HS waitlisted pts (22 controls).
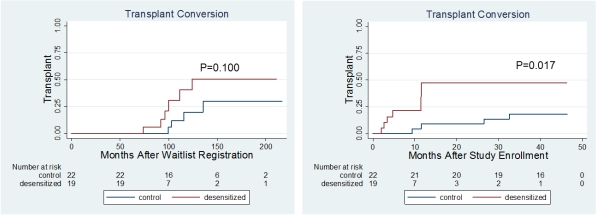
Mean age of DES group was 49.6±11.7 years old. Male to female ratio was 10:9. Baseline mean cPRA values were 82 ± 26%. Baseline mean class I and II MFI values were 1,837 ± 1,966 and 1,832 ± 1,048. Baseline peak class I and II MFI values were 12,875 ± 5,047 and 9,495 ± 7,201. After DES, KT were successfully performed in 6 pts (31.6%), compared to 4 (18.2%) pts among non-DES controls (p=0.017). Age and gender adjusted time varying covariate Cox analysis showed that DES increased the probability of receiving KT ( hazard ratio of 28.306 (95% C.I. 5.574 – 143.759)). However, neither cPRA values nor mean MFI values were changed. In the transplanted group, pre-DES donor specific antibody (DSA) levels were below 2,000 MFI in 5 of 6 pts, and DES decreased DSA level only in 1 patient from 9,860 to 7,125 MFI. DES did not significantly decrease flowcytometric crossmatch intensity. DES protocol was well tolerated without serious adverse event, and 94.7% pts completed the protocol. No acute rejection occurred in DES KT group, whereas it occurred in 2 patients (50%) in non-DES KT group.
DES including concomitant bortezomib and rituximab reduced neither cPRA nor mean MFI values. However, DES increased the probability of DDKT in HS waitlisted pts, and post-KT outcomes were good.
To cite this abstract in AMA style:
Kang S, Lim H, Jeong J, Song E, Yun I, Ahn C, Yang J. Desensitization Protocol Including Concomitant Bortezomib and Rituximab Did Not Reduce Calculated PRA Nor Mean MFI Values Among Waitlisted Patients for Deceased Donor Kidney Transplantation [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/desensitization-protocol-including-concomitant-bortezomib-and-rituximab-did-not-reduce-calculated-pra-nor-mean-mfi-values-among-waitlisted-patients-for-deceased-donor-kidney-transplantation/. Accessed July 15, 2025.« Back to 2015 American Transplant Congress
