Desensitization for Deceased Donor Transplantation: Testing a New Paradigm in the Nonhuman Primate
Duke University, Durham, NC
Meeting: 2020 American Transplant Congress
Abstract number: 541
Keywords: Alloantibodies, Co-stimulation, Sensitization, T helper cells
Session Information
Session Name: B-cells / Antibodies /Autoimmunity
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:27pm-3:39pm
Presentation Time: 3:27pm-3:39pm
Location: Virtual
*Purpose: Desensitization regimens have rarely been applied to deceased donor allotransplantation. We hypothesized that belatacept monotherapy during a simulated deceased donor waiting list period would improve graft survival in sensitized nonhuman primates desensitized with carfilzomib and belatacept prior to the waiting list period.
*Methods: Rhesus macaques (n=7) were sensitized to maximally MHC-mismatched donors with two sequential skin transplants, then desensitized with carfilzomib and belatacept. This was followed by a simulated waiting list period of 3 months, during which time 3 animals received belatacept monotherapy (Desensitization + Prolonged Belatacept) and 4 received no additional treatment (Desensitization Alone). Animals then received life-sustaining kidney transplants from their MHC-mismatched skin donors and received rhesus-specific antithymocyte globulin induction and standard triple immunosuppression. One animal was excluded from post-transplant analysis due to a technical complication.
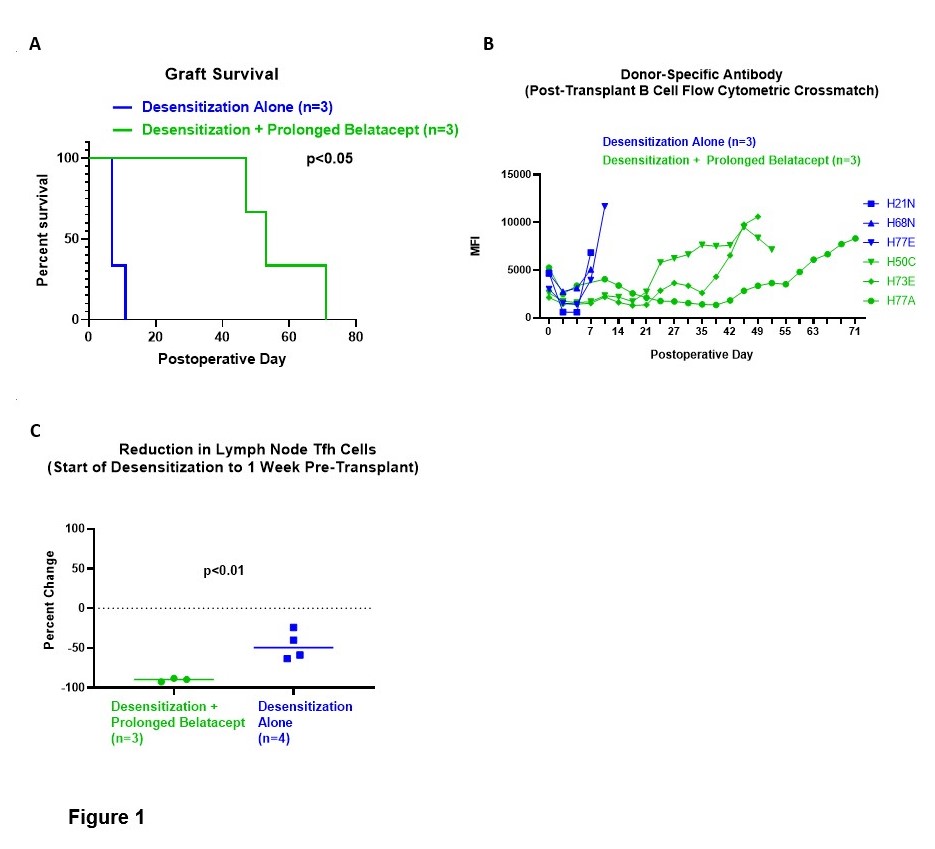
*Results: Compared to animals treated with Desensitization Alone, animals treated with Desensitization + Prolonged Belatacept showed improved graft survival (p<0.05, Figure 1A), a delayed post-transplant rebound in donor-specific antibody (Figure 1B), and a more profound reduction of lymph node Tfh cells during the pre-transplant treatment period (p<0.01, Figure 1C). Tfh cells repopulated after belatacept cessation and kidney transplantation (p<0.05, not shown).
*Conclusions: Belatacept monotherapy during a simulated waitlist period was associated with improved graft survival in previously desensitized nonhuman primates and suggests a strategy for applying clinical desensitization regimens to deceased donor transplantation. Post-transplant rebound of both donor-specific antibody levels and Tfh cells suggest that alternative maintenance immunosuppression regimens that better control the germinal center response may further improve outcomes.
To cite this abstract in AMA style:
Fitch ZW, Schmitz R, Schroder PM, Manook M, Choi AY, Yoon J, Kwun J, Knechtle SJ. Desensitization for Deceased Donor Transplantation: Testing a New Paradigm in the Nonhuman Primate [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/desensitization-for-deceased-donor-transplantation-testing-a-new-paradigm-in-the-nonhuman-primate/. Accessed March 1, 2026.« Back to 2020 American Transplant Congress