Decay Accelerating Factor (DAF) Regulates Germinal Center B-cell and Alloantibody Formation
1Nephrology, Mount Sinai School of Medicine, New York, NY, 2Tisch Cancer Institute, Mount Sinai School of Medicine, New York, NY, 3Medicine, Endocrinology, Diabetes and Bone Disease, Mount Sinai School of Medicine, New York, NY, 4Clinical Immunology, Mount Sinai School of Medicine, New York, NY
Meeting: 2019 American Transplant Congress
Abstract number: 168
Keywords: Alloantibodies, B cells, Mice, knockout, Sensitization
Session Information
Session Name: Concurrent Session: B-cell / Antibody /Autoimmunity
Session Type: Concurrent Session
Date: Sunday, June 2, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:54pm-5:06pm
Presentation Time: 4:54pm-5:06pm
Location: Room 310
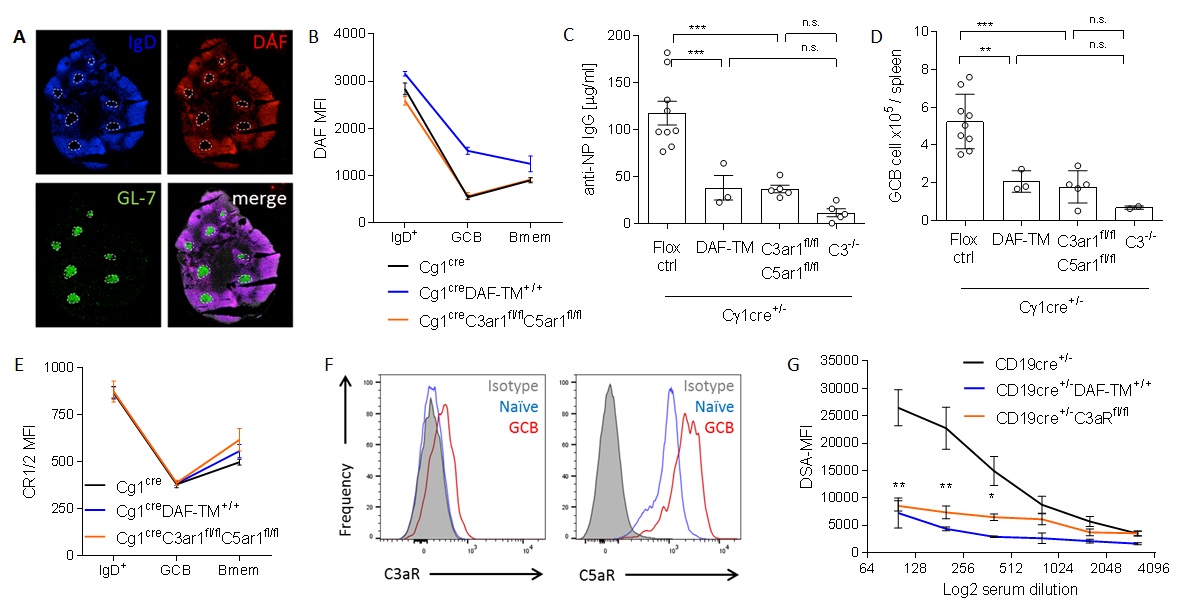
*Purpose: High affinity IgG antibodies including alloantibodies result from germinal center (GC) reactions in which somatic hypermutation and positive selection cause affinity maturation. When we compared cell surface molecule expression profiles of murine (A,B) and human naïve, memory and GC B cells we observed that GC B cells specifically lacked the complement regulator DAF, raising the possibility that DAF down-regulation on GC B cells is linked to GC function.
*Methods: To test this hypothesis, we constructed a mouse in which a transmembrane spanning form of DAF is conditionally overexpressed as a transgene (DAF-TM+/+) and crossed it to a Cgamma1 (Cg1) Cre-expressing mouse such that the DAF transgene is only expressed when B cells enter the GC (B).
*Results: Immunization with the model antigen NP or with allogeneic cells resulted in significantly reduced titers of antigen specific IgG by 3x fold in the DAF-TM+/+ animals vs. controls (C). Analyses of GCs confirmed DAF downregulation on control GC B cells but persistent DAF expression on DAF-TM+/+ mice (B), and demonstrated 3x fold fewer GC B cells by flow cytometry (D). Surface expression of other complement regulators and receptors, including CR2 known to be involved in B cell activation, did not differ between genotypes (E). Because DAF regulates C3 convertases and DAF deficiency augments C3a and C5a production, we tested whether C3a/C3aR and C5a/C5aR ligations on GC B cells is essential for their function. Flow cytometry showed that C3aR and C5aR expression are upregulated on GC B cells vs naïve B cells (F). We next generated mice conditionally deficient in both C3aR and C5aR, and crossed them to the Cg1-Cre mice such that both receptors are specifically deleted GC B cells. Absence of GC B cell C3aR/C5aR prevented antibody formation and markedly reduced GC B cell differentiation following immunization with NP (C,D) or allogeneic cells (G), newly demonstrating that C3aR/C5aR signaling on GC B cells is required for GC function.
*Conclusions: Together, our data show that DAF down-regulation on GC B cells facilitates C3aR/C5aR signaling required for GC function. In addition to delineating a paradigm shifting role for DAF and complement as regulators of GC dynamics, the findings have therapeutic implications for preventing and treating alloantibody formation in transplant recipients.
To cite this abstract in AMA style:
Cumpelik A, Varano G, Heja D, Homann D, Lira S, Dominguez-Sola D, Heeger PS. Decay Accelerating Factor (DAF) Regulates Germinal Center B-cell and Alloantibody Formation [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/decay-accelerating-factor-daf-regulates-germinal-center-b-cell-and-alloantibody-formation/. Accessed February 15, 2026.« Back to 2019 American Transplant Congress