De Novo Malignancy Occurrence After Kidney Transplantation in HLA-Sensitized Patients Treated with B-Cell Targeting Therapy.
1CHU de Bordeaux, Bordeaux, France
2Université
de Bordeaux, Bordeaux, France
3UMR CNRS 5164 Immunoconcept, Bordeaux, France
4Clinique Saint-Augustin-CTMR, Bordeaux, France
Meeting: 2017 American Transplant Congress
Abstract number: B177
Keywords: Antibodies, Induction therapy, Malignancy, Rejection
Session Information
Session Name: Poster Session B: Kidney Immunosuppression: Induction Therapy
Session Type: Poster Session
Date: Sunday, April 30, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Management of high risk transplantations in patients with preformed DSA still remains a dilemma. Many groups propose to increase the immunosuppression in these patients by adding a B-cell targeting therapy (rituximab + intravenous immunoglobulin (IVIG)) in order to reduce the incidence and magnitude of HLA antibody rebound, and prevent antibody-mediated rejection (AMR). However, the impact of rituximab/IVIG on the incidence of post-transplant de novo malignancy is still unknown. The goal of this study was to determine whether rituximab/IVIG is associated with a higher incidence of de novo malignancy in sensitized patients.
48 kidney recipients transplanted with preformed DSA received an induction therapy associating thymoglobulin/IVIG/rituximab (375mg/m2) (RTX/IVIG group). They were compared to a control group (CTRL group) of 154 sensitized patients receiving thymoglobulin alone. 90% of the patients were treated with tacrolimus and mycophenolate mofetil.
Thirty-nine out of 202 (19.3%) patients developed de novo malignancy without any difference between the RTX/IVIG and CTRL groups [7 (14.6%) vs 32 (20.8%), respectively, p=0.3]. Distribution was similar between the two groups with a majority of non-melanoma skin cancer (NMSC, n=24). Identified risk factors for de novo malignancy occurrence were male gender, age, a past medical history of cancer and azathioprine-maintenance therapy. Noteworthy, we observed an excellent graft survival profile in patients who experienced at least one NMSK because none of them had AMR or graft loss during the follow-up (figure).
In conclusion, our results support the safety of B-cells targeting therapy based on IVIG and RTX association, regarding the occurrence of de novo malignancy in patients transplanted with preformed DSA. Moreover, occurrence of NMSC could reflect a state of over immunosuppression in sensitized patients associated with an excellent graft survival.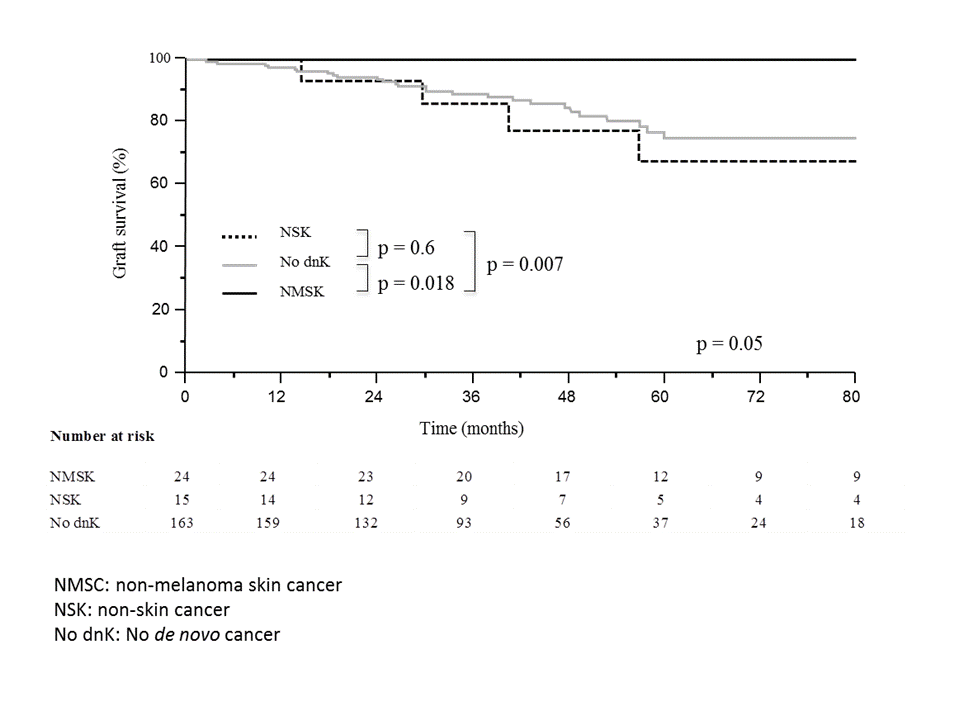
CITATION INFORMATION: Couzi L, Davis P, Visentin J, Taton B, Guidicelli G, Kaminski H, Merville P, Bachelet T. De Novo Malignancy Occurrence After Kidney Transplantation in HLA-Sensitized Patients Treated with B-Cell Targeting Therapy. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Couzi L, Davis P, Visentin J, Taton B, Guidicelli G, Kaminski H, Merville P, Bachelet T. De Novo Malignancy Occurrence After Kidney Transplantation in HLA-Sensitized Patients Treated with B-Cell Targeting Therapy. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/de-novo-malignancy-occurrence-after-kidney-transplantation-in-hla-sensitized-patients-treated-with-b-cell-targeting-therapy/. Accessed February 21, 2026.« Back to 2017 American Transplant Congress
