De Novo Low-Dose Sirolimus versus Mycophenolate Mofetil in Combination with Extended-Release Tacrolimus in Kidney Transplant Recipients.
1Transplantation Surgery, Severance Hospital, Yonsei University Health System, Seoul, Republic of Korea
2Surgery, Seoul National University Hospital, Seoul, Republic of Korea
3Surgery, Ajou University Hospital, Suwon, Republic of Korea
4Surgery, Gangnam Severance Hospital, Yonsei University Health System, Seoul, Republic of Korea
5Internal Medicine, Kyungpook National University Hospital, Daegu, Republic of Korea
6Surgery, Ulsan University Hospital, Ulsan, Republic of Korea
7Surgery, Korea University Anam Hospital, Seoul, Republic of Korea
8Pathology, Yonsei University College of Medicine, Seoul, Republic of Korea
Meeting: 2017 American Transplant Congress
Abstract number: 72
Keywords: Adverse effects, Graft survival, Immunosuppression, Kidney transplantation
Session Information
Session Name: Concurrent Session: Novel Immunosuppression Regimens - Tacrolimus Combinations
Session Type: Concurrent Session
Date: Sunday, April 30, 2017
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:18pm-3:30pm
Presentation Time: 3:18pm-3:30pm
Location: E450b
Previous studies reported that tacrolimus (TAC) with high-dose sirolimus (SRL) was associated with worse outcomes in kidney transplantation, compared to TAC with mycophenolate mofetil (MMF.). However, outcomes using low-dose SRL with TAC are uncertain. We aimed to assess the efficacy and safety of low-dose SRL with extended-release tacrolimus (ER-TAC) versusMMF with ER-TAC.
We randomly assigned 158 renal transplant patients to receive low-dose SRL or MMF in combination with ER-TAC and corticosteroid. The primary endpoint was the composite efficacy failure rate, including biopsy-proven acute rejection (BPAR), graft loss, death, or loss to follow-up, within 12 months post-transplantation.
The efficacy failure rate was 6.6% in the low-dose SRL group and 13.3% in the MMF group in the intention-to-treat population (absolute difference, 6.8%; 95% CI -2.8 to 16.3%). The incidence of BPAR within 12 months post-transplantation was 5.3% in the low-dose SRL group and 13.3% in the MMF group (p=0.09). The mean estimated glomerular filtration rate, the incidences of adverse events and serious adverse events were similar between groups. Low-dose SRL with ER-TAC was not inferior to MMF with ER-TAC with respect to efficacy and safety. Low-dose SRL with ER-TAC can effectively prevent acute rejection and preserve renal function. (ClinicalTrial.gov number, NCT01680952.)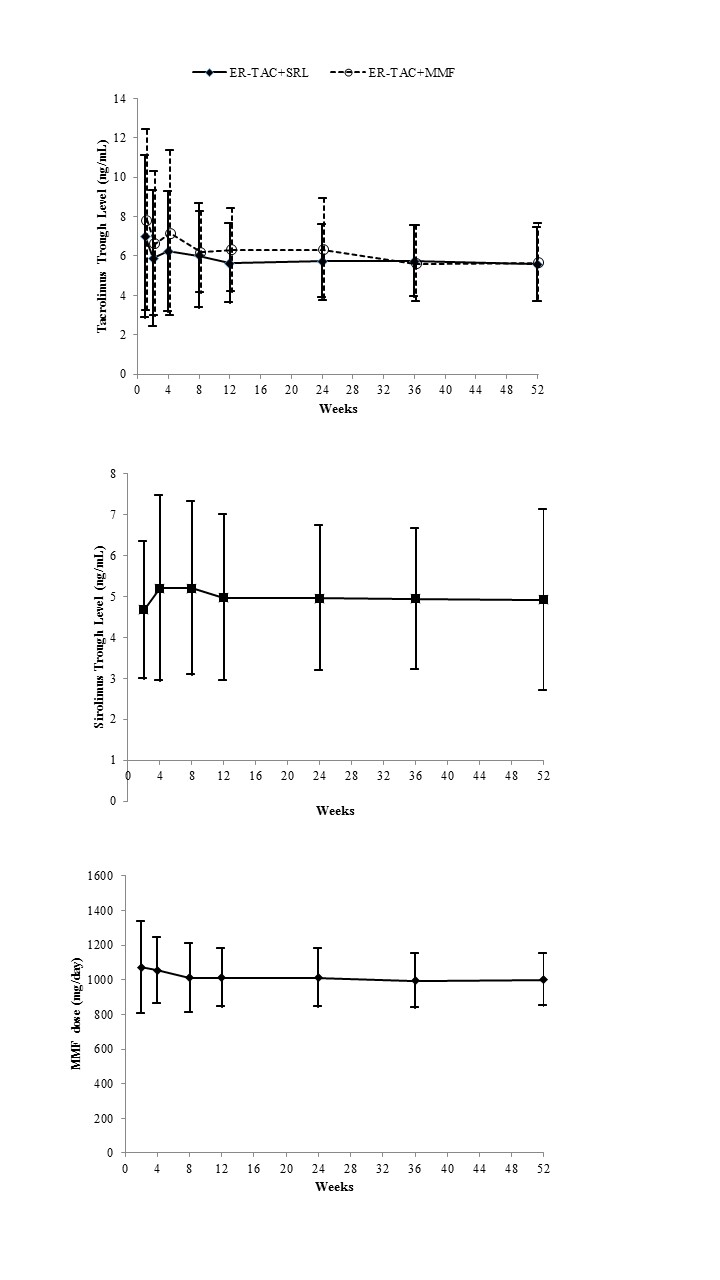
CITATION INFORMATION: Lee J, Huh K, Ha J, Oh C.-K, Ju M, Kim C.-D, Cho H, Jung C, Lim B, Kim Y. De Novo Low-Dose Sirolimus versus Mycophenolate Mofetil in Combination with Extended-Release Tacrolimus in Kidney Transplant Recipients. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Lee J, Huh K, Ha J, Oh C-K, Ju M, Kim C-D, Cho H, Jung C, Lim B, Kim Y. De Novo Low-Dose Sirolimus versus Mycophenolate Mofetil in Combination with Extended-Release Tacrolimus in Kidney Transplant Recipients. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/de-novo-low-dose-sirolimus-versus-mycophenolate-mofetil-in-combination-with-extended-release-tacrolimus-in-kidney-transplant-recipients/. Accessed February 18, 2026.« Back to 2017 American Transplant Congress
