Daratumumab Therapy Achieves Dialysis Independence in Kidney Transplant Recipients with Resistant Proliferative Glomerulonephritis with Monoclonal Immunoglobulin Deposits
The Ohio State University, Columbus, OH
Meeting: 2022 American Transplant Congress
Abstract number: 1426
Keywords: Glomerulonephritis, Immunoglobulins (Ig), Recurrence, Renal dysfunction
Topic: Clinical Science » Kidney » 49 - Recurrent Kidney Disease & Genetics
Session Information
Session Name: Recurrent Kidney Disease & Genetics
Session Type: Poster Abstract
Date: Monday, June 6, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID) is a unique cause for monoclonal gammopathy of renal significance with overall poor prognosis. Patients are frequently treated with plasma-cell targeted therapies. Daratumumab is an anti-CD38 monoclonal antibody that depletes plasma cells. It has been used for treatment of PGNMID in native kidney disease with mixed results.
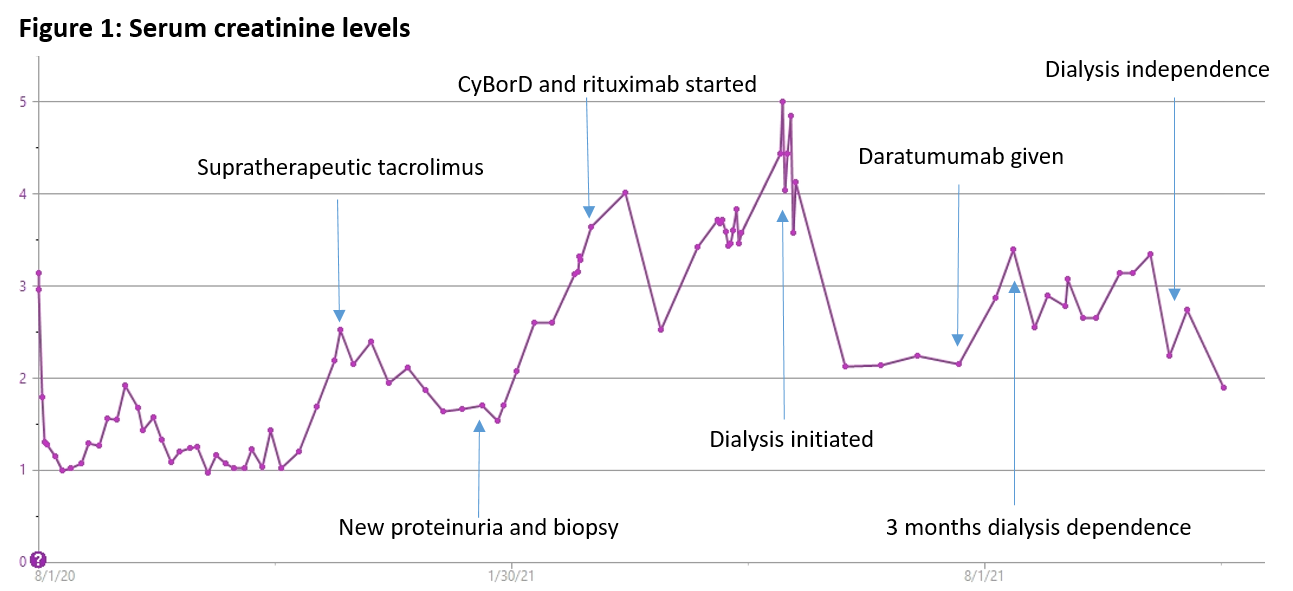
*Methods: 70 year old Caucasian female with past medical history of end stage kidney disease from presumed diabetic nephropathy underwent a deceased donor kidney transplant in August 2020. She received induction with thymoglobulin and maintenance with mycophenolic acid and tacrolimus. Immediate post-operative course was uncomplicated with baseline serum creatinine (sCr) of 1.1mg/dL. Six months post-transplant, new nephrotic range proteinuria was noted with urine protein to creatinine ratio of 11. Renal function was otherwise stable with sCr of 1.2 mg/dL. Kidney transplant biopsy was consistent with PGNMID with dominant IgG2 lambda deposits. There was no detectable monoclonal protein in the serum or urine. Free light chain ratio was normal. Bone marrow biopsy revealed normocellular bone marrow with mildly increased, polytypic plasma cells (2-3%) without light chain restriction. Patient was initially treated with first cycle of cyclophosphamide, bortezomib, and dexamethasone (CyBorD), but had progression of renal dysfunction with refractory volume overload. Subsequently, therapy was changed to rituximab. However, renal function continued to deteriorate requiring a return to dialysis nine months post-transplant. After a multidisciplinary discussion, the decision was made to attempt therapy with daratumumab (16 mg/kg) once weekly infusion for eight weeks, followed by every other week for eight additional weeks. Maintenance immunosuppression with tacrolimus and prednisone was continued.
*Results: After eight weekly daratumumab infusions, the patient’s renal function started to improve. At 14 months post-transplant, she was liberated from dialysis after being dialysis-dependent for over five months. On the last follow up, proteinuria had improved to 1.2 g and sCr was in the range of 1.9-2.2 mg/dL.
*Conclusions: Daratumumab appears to be a safe and effective treatment for PGNMID in kidney transplant recipients. It could be considered as a therapeutic agent even in patients with severe renal allograft dysfunction requiring dialysis treatment.
To cite this abstract in AMA style:
Yenebere P, Daloul R. Daratumumab Therapy Achieves Dialysis Independence in Kidney Transplant Recipients with Resistant Proliferative Glomerulonephritis with Monoclonal Immunoglobulin Deposits [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/daratumumab-therapy-achieves-dialysis-independence-in-kidney-transplant-recipients-with-resistant-proliferative-glomerulonephritis-with-monoclonal-immunoglobulin-deposits/. Accessed March 3, 2026.« Back to 2022 American Transplant Congress