Daratumumab for Recurrence of Proliferative Glomerulonephritis with Monoclonal Immunoglobulin Deposits in a Post-Kidney Transplant Recipient: A Case Report
1Transplant, Barnes-Jewish Hospital, St. Louis, MO, 2Washington University School of Medicine at St. Louis, Saint Louis, MO, 3Transplant, Washington University, St. Louis, MO, 4Washington University School of Medicine in St. Louis, Saint Louis, MO, 5Washington University School of Medicine, Saint Louis, MO, 6Washington University, Saint Louis, MO, 7Transplant Nephrology, Washington University School of Medicine, St. Louis, MO, 8Transplant, Washington University School of Medicine, Saint Louis, MO
Meeting: 2022 American Transplant Congress
Abstract number: 1423
Keywords: Glomerulonephritis, Kidney transplantation, Monoclonal antibodies
Topic: Clinical Science » Kidney » 49 - Recurrent Kidney Disease & Genetics
Session Information
Session Name: Recurrent Kidney Disease & Genetics
Session Type: Poster Abstract
Date: Monday, June 6, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Proliferative Glomerulonephritis with Monoclonal Immunoglobulin Deposits (PGNMID) is a rare kidney disease associated with a high rate of recurrence post-kidney transplantation resulting in graft loss. PGNMID has variable response rates to immunosuppression. Daratumumab is an anti-CD38 monoclonal antibody that has recently demonstrated a benefit for complete or partial remission in pre-transplant PGNMID patients. To date, there has been no reported daratumumab use for PGNMID in a post-kidney transplant recipient.
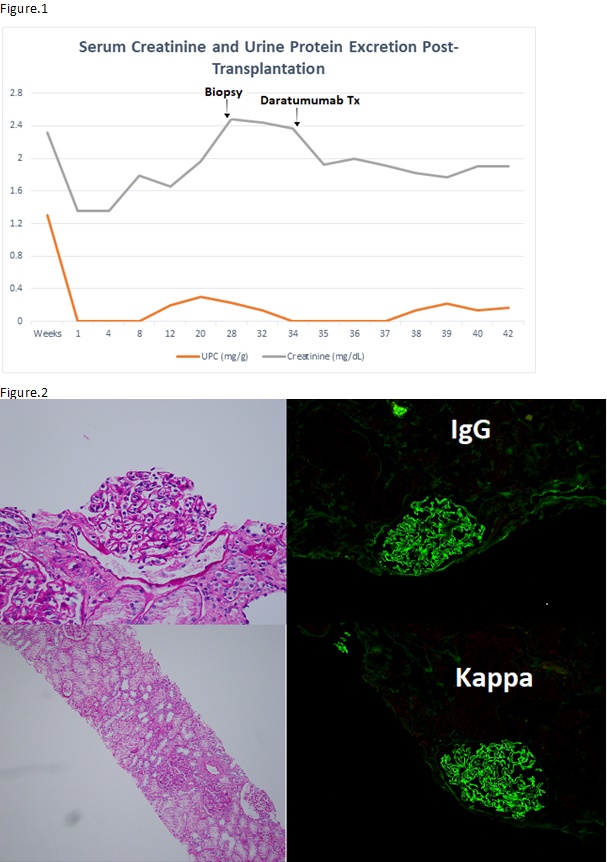
*Methods: We present a case of a 30-year-old male with a history of end stage renal disease (ESRD) secondary to PGNMID previously unresponsive to immunosuppressive therapies including steroids, rituximab, mycophenolate and bortezomib. He experienced biopsy-proven recurrence of PGNMID approximately 7 months after receiving a deceased donor kidney transplant. Figure.1 shows the trend of serum creatinine (Scr) and urine protein excretion post-kidney transplant. Patient had increase in Scr up to 2.6 mg/dL from 1.4mg/dL along with microscopic hematuria and proteinuria (0.324). The biopsy demonstrated diffuse endocapillary hypercellularity and monoclonal IgG kappa, serum immunofixation was negative for monoclonal proteins. (Figure.2)
*Results: Due to the patient’s previous unresponsiveness the decision was made to treat with daratumumab starting 34 weeks post-transplant at labeled dosing of 16 mg/kg intravenous weekly for 8 doses followed by 16 mg/kg every 2 weeks for 8 doses. Within a week of treatment, a downtrend in Scr was observed from 2.6 mg/dL to a new baseline of 1.7 md/dL on last follow-up. The patient experienced a mild infusion reaction with the first infusion but tolerated all subsequent infusions. The patient was placed on antiviral prophylaxis for the duration of treatment and did not experience infection during the course of treatment.
*Conclusions: This is the first case of the use of daratumumab demonstrating safety and efficacy against recurrent PGNMID in a kidney transplant recipient.
To cite this abstract in AMA style:
Progar K, Alhamad T, Murad H, Java A, Malone A, Gaut J, Santos RDelos, Merzkani M. Daratumumab for Recurrence of Proliferative Glomerulonephritis with Monoclonal Immunoglobulin Deposits in a Post-Kidney Transplant Recipient: A Case Report [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/daratumumab-for-recurrence-of-proliferative-glomerulonephritis-with-monoclonal-immunoglobulin-deposits-in-a-post-kidney-transplant-recipient-a-case-report/. Accessed February 20, 2026.« Back to 2022 American Transplant Congress