Cytomegalovirus Prophylaxis in Pediatric Liver Transplantation: A Comparison of Strategies Across the SPLIT Consortium
E. D. Knackstedt1, S. G. Anderson2, R. Anand2, J. Mitchell2, J. Bucuvalas3, L. Danziger-Isakov4
1Univ of Utah/Primary Children's Hosp, SLC, UT, 2Emmes, Rockville, MD, 3Icahn School of Med at Mount Sinai, New York City, NY, 4Cincinnati Children's Hosp Med Ctr, Cincinnati, OH
Meeting: 2022 American Transplant Congress
Abstract number: 1343
Keywords: Cytomeglovirus, Liver transplantation, Pediatric
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis) III
Session Type: Poster Abstract
Date: Monday, June 6, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: To compare the effectiveness of short (<120d) v. long (>180d) cytomegalovirus (CMV) prophylaxis in pediatric liver transplantation (pLTX).
*Methods: A prospective cohort study of primary pLTX (≤18 yrs of age) recipients enrolled in the Society of Pediatric Liver Transplantation (SPLIT) registry with either donor or recipient CMV seropositivity at transplant from 2015-19. Participants were grouped into the short or long prophylaxis based on their center’s practice and intended duration. Centers prospectively collected antiviral duration, adverse events (neutropenia, acute kidney injury), CMV viral testing and episodes, adjuvant therapies, and outcomes including graft and patient survival at 3m intervals for the 1st 15m post-pLTX.
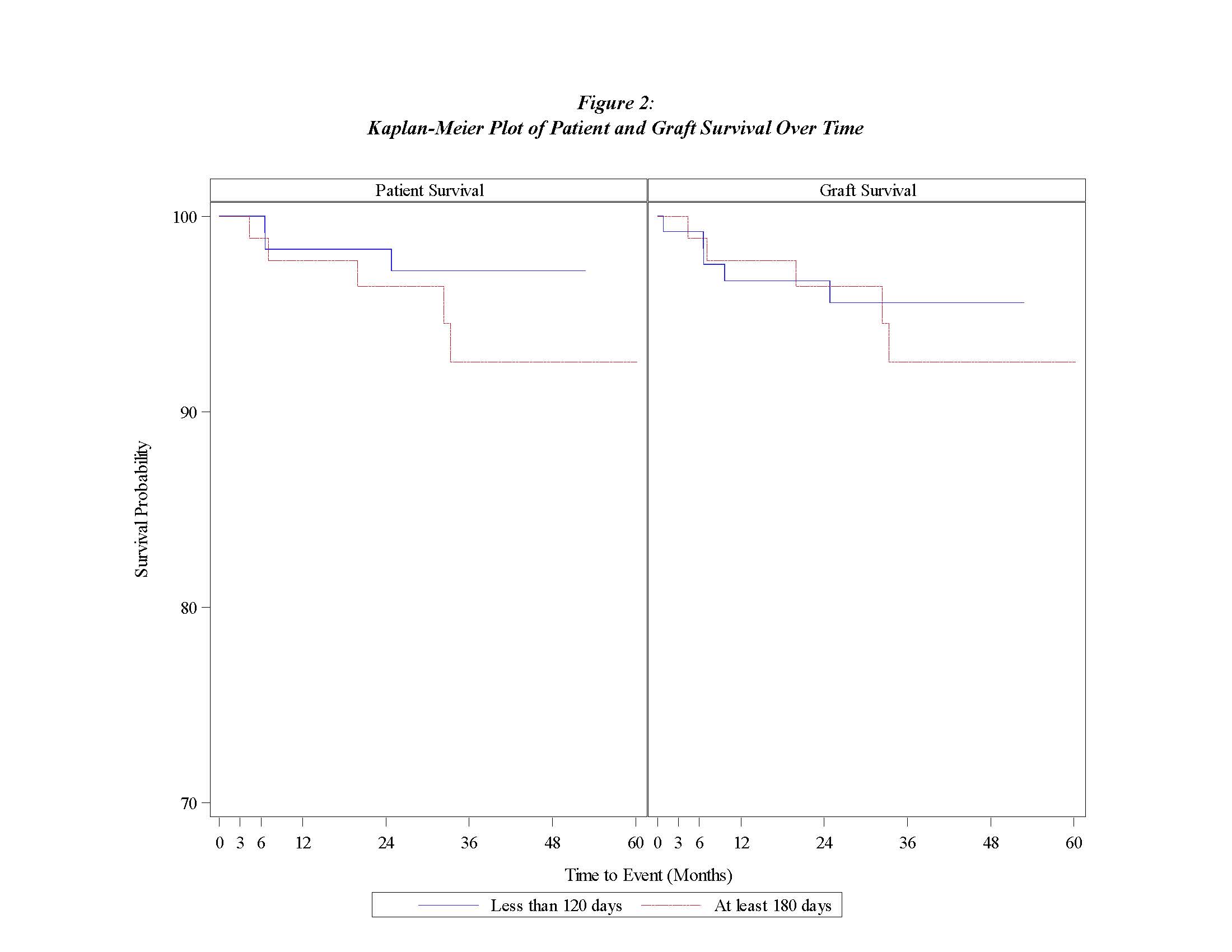
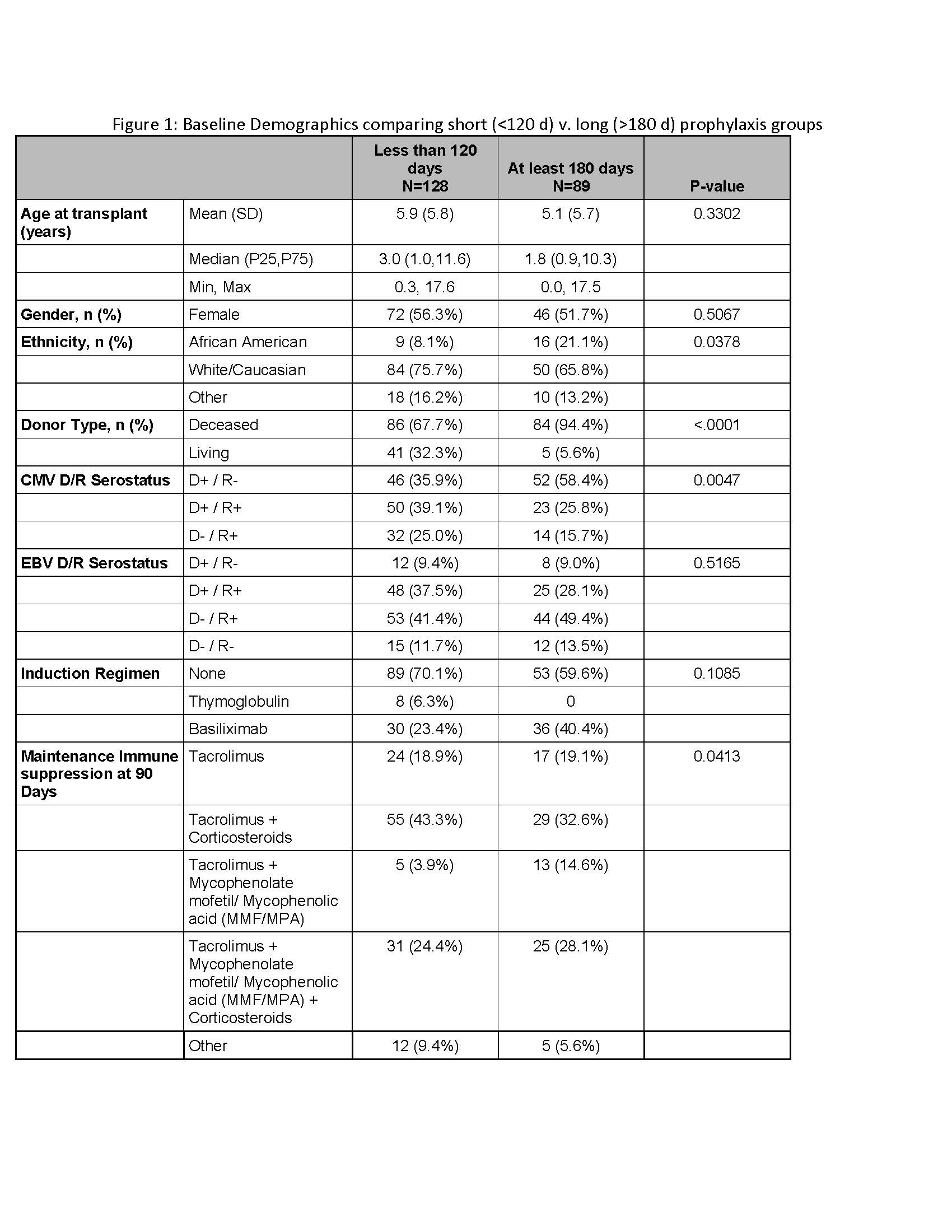
*Results: 217 pLTX recipients were enrolled including 128 (59%) short and 89 (41%) long prophylaxis groups. Age, gender, EBV serostatus, and induction immunosuppression were similar between the groups. However, the long group was more likely to be African-American, have a deceased donor, and be CMV high risk (D+R-); maintenance immunosuppression differed as well (Fig. 1). The median duration of antiviral prophylaxis received was 54 days (IQR 13-105) in the short group and 177 days (IQR 97-226) in the long group. While CMV syndrome was more common in the short group (14.1% v. 4.5%; p=0.02), end-organ disease and CMV DNAemia events (asymptomatic CMV) were similar (2.3% and 1.1%; p=0.51 and 19.5% v. 14.6%; p=0.35) Neutropenia was more common in long group (16.2% v. 55.2%; p<0.0001), but acute kidney injury was similar between the groups (13.4% v. 20.7%; p=0.17). Graft and patient survival were similar among the groups (Fig. 2).
*Conclusions: For both short and long prophylaxis groups, CMV end-organ disease was rare and graft and patient survival were similar. However, the long group experienced less CMV syndrome, but more neutropenia during prophylaxis. Therefore, consideration of a short prophylaxis duration must weigh potential increased risk of CMV syndrome against less medication burden and neutropenia.
To cite this abstract in AMA style:
Knackstedt ED, Anderson SG, Anand R, Mitchell J, Bucuvalas J, Danziger-Isakov L. Cytomegalovirus Prophylaxis in Pediatric Liver Transplantation: A Comparison of Strategies Across the SPLIT Consortium [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/cytomegalovirus-prophylaxis-in-pediatric-liver-transplantation-a-comparison-of-strategies-across-the-split-consortium/. Accessed March 1, 2026.« Back to 2022 American Transplant Congress