Costimulation Independent Acute Rejection Requires CD127 Signaling.
Department of Surgery, Emory University, Atlanta, GA
Meeting: 2017 American Transplant Congress
Abstract number: 559
Keywords: Co-stimulation, Graft survival, T cells
Session Information
Session Time: 8:00am-10:00am
 Presentation Time: 8:15am-8:30am
Presentation Time: 8:15am-8:30am
Location: Arie Crown Theater
Clinical costimulation blockade with belatacept leads to improved survival and graft function. However, a subset of patients experience costimulation independent rejection. We identified a critical threshold of CD28hi memory T cells which predicted costimulation independent rejection in humans (n=10) and non-human primates (n=31) with an 100% and 90.9% positive predictive value respectively. This CD28hi memory subset uniquely express higher levels of IL-7Rα, CD127 compared to CD28lo memory cells.  Using the stringent Balb/C to C57BL/6 skin allotransplantation model, we investigated CD127 expression kinetics. At the peak of rejection two distinct populations arise: short lived effectors (CD127lo KLRG1hi) and memory precursors (CD127hi KLRG1lo). While costimulation blockade delays the emergence of short lived effectors, it fails to curtail the development of memory precursors. All animals eventually rejected under costimulation blockade based immunosuppression (MST=21 days). The addition of anti-CD127 prevented the development of acute allograft rejection and led to indefinite survival (p<.0001, MST>80 days,
Using the stringent Balb/C to C57BL/6 skin allotransplantation model, we investigated CD127 expression kinetics. At the peak of rejection two distinct populations arise: short lived effectors (CD127lo KLRG1hi) and memory precursors (CD127hi KLRG1lo). While costimulation blockade delays the emergence of short lived effectors, it fails to curtail the development of memory precursors. All animals eventually rejected under costimulation blockade based immunosuppression (MST=21 days). The addition of anti-CD127 prevented the development of acute allograft rejection and led to indefinite survival (p<.0001, MST>80 days, 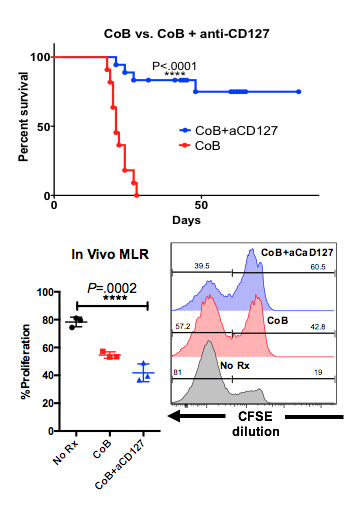 ). We investigated the impact of CD127 blockade on allostimulated T cell proliferation. CFSE labeled C57BL/6 splenocytes were transferred to irradiated Balb/C recipients, who were then treated with costimulation blockade, or combined costimulation blockade + anti-CD127, or received no therapy. The addition of anti-CD127 to costimulation blockade significantly abrogated allostimulated proliferative response, nearly reducing proliferation by half (p=.0002, Fig 2). Taken together these data suggest that CD127 signaling is required for costimulation independent rejection by providing critical proliferative signals for alloreactive T cells.
). We investigated the impact of CD127 blockade on allostimulated T cell proliferation. CFSE labeled C57BL/6 splenocytes were transferred to irradiated Balb/C recipients, who were then treated with costimulation blockade, or combined costimulation blockade + anti-CD127, or received no therapy. The addition of anti-CD127 to costimulation blockade significantly abrogated allostimulated proliferative response, nearly reducing proliferation by half (p=.0002, Fig 2). Taken together these data suggest that CD127 signaling is required for costimulation independent rejection by providing critical proliferative signals for alloreactive T cells.
CITATION INFORMATION: Mathews D, Dong Y, Kim S, Breeden C, Adams A. Costimulation Independent Acute Rejection Requires CD127 Signaling. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Mathews D, Dong Y, Kim S, Breeden C, Adams A. Costimulation Independent Acute Rejection Requires CD127 Signaling. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/costimulation-independent-acute-rejection-requires-cd127-signaling/. Accessed February 24, 2026.« Back to 2017 American Transplant Congress
