Conversion of Tacrolimus Immediate Release to LCP Tacrolimus in Obese Kidney Transplant Recipients
MUSC, Charleston, SC
Meeting: 2022 American Transplant Congress
Abstract number: 533
Keywords: Adverse effects, Dosage, Kidney transplantation, Obesity
Topic: Clinical Science » Pharmacy » 29 - Non-Organ Specific: Pharmacokinetics / Pharmacogenomics / Drug interactions
Session Information
Session Name: Non-Organ Specific: Pharmacokinetics / Pharmacogenomics / Drug interactions
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:40pm-6:50pm
Presentation Time: 6:40pm-6:50pm
Location: Hynes Room 311
*Purpose: Data describing the safety, efficacy and conversion ratio of tacrolimus immediate release (IR) to LCP-tacrolimus (LCP) in obese patients (BMI > 30) is lacking. We sought to assess the conversion of tacrolimus IR to LCP-tacrolimus in kidney transplant recipients and determine if the standard conversion of 1:0.8 is appropriate.
*Methods: This was a retrospective longitudinal cohort study including patients converted to LCP from June 2019 to October 2020 and were followed until November 2021. The primary outcome was conversion ratio based on weight-based dose at a stable therapeutic level, defined as the first level within goal range without a dose change. Other outcomes assessed were tacrolimus coefficient of variation (CV), time in therapeutic range, adverse event rates, infection (BK, CMV), development of donor specific antibodies, and biopsy-proven acute rejection. Categorical data were analyzed with chi square. Continuous variables were assessed using paired t-test and one-way ANOVA.
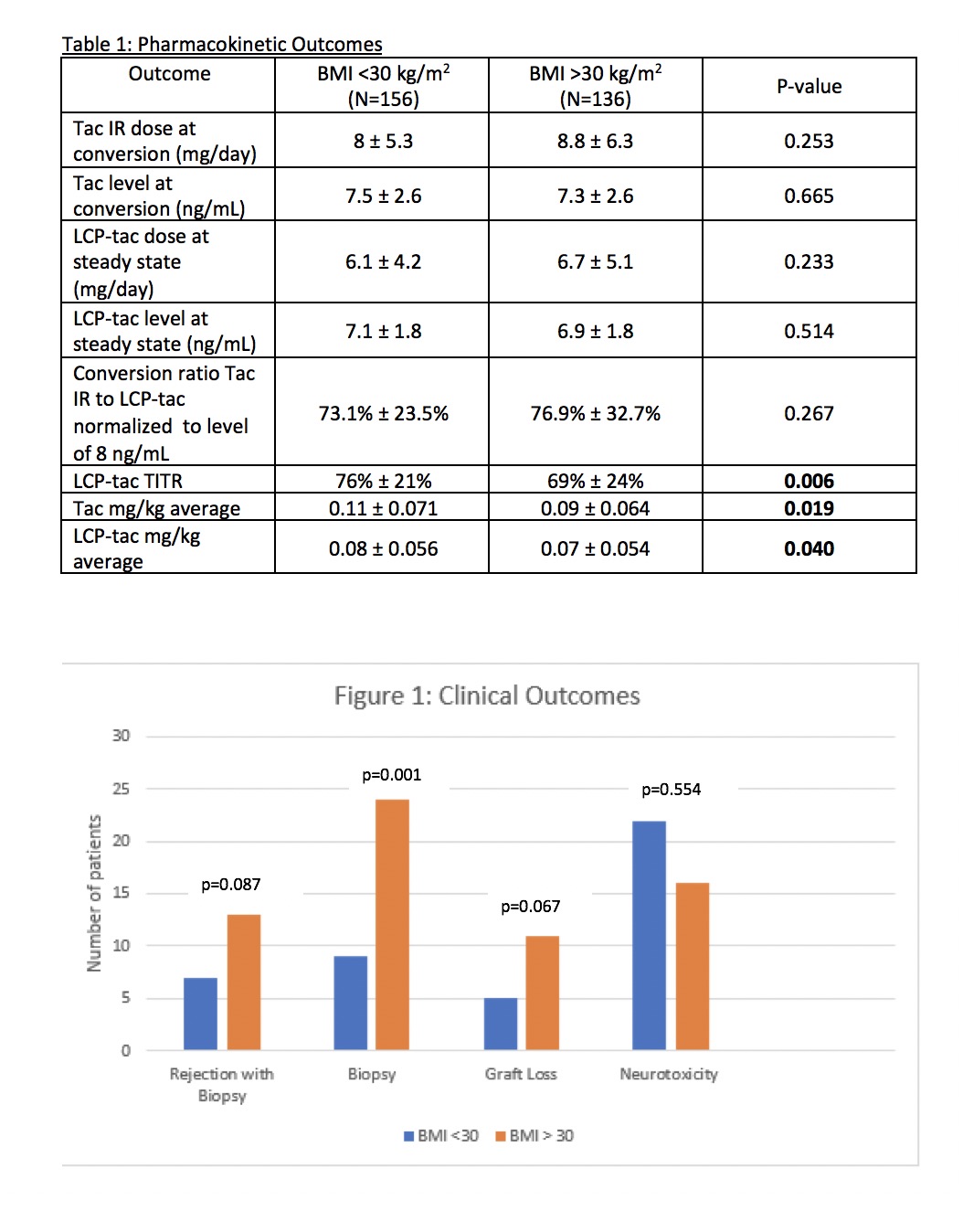
*Results: A total of 292 patients were included; 156 and 136 patients with a BMI <30 and BMI >30, respectively. Baseline characteristics were similar, with the exception of pancreas transplant (7.7% vs 2.2%), diabetes (51.9% vs 66.9%), and HLA mismatch (4.3 vs 3.9), respectively. The dosing ratio from tac IR to LCP ranged from 0.73 to 0.77, which differs compared to the package insert recommendation of 0.80; mean LCP dose was similar between BMI cohorts (0.08 vs 0.07 for BMI <30 and BMI > 30, respectively). Overall TITR, defined as percentage of time in therapeutic range for duration of follow up for LCP, was 76% vs 69% for BMI <30 and >30, respectively (p=0.006). In multivariable modeling, BMI was a significant predictor for LCP-tacrolimus dose at steady state; for every 1 kg/m2 increase in BMI, patients needed 0.03 mg/kg less of LCP. Rejection and graft loss were slightly higher in the BMI >30 kg/m2, although not reaching statistical significance.
*Conclusions: These findings demonstrate that patients with a BMI >30 kg/m2 had slightly lower LCP-tacrolimus mg/kg dosing, which may be due to lower TITR. Further research is needed to assess if high BMI is a risk-factor for reduced tac tolerability.
To cite this abstract in AMA style:
Newman J, Patel N, Patel S, Sprague T, Andrade E, Rao N, Bartlett F, DuBay D, Rohan V, Casey M, Taber D. Conversion of Tacrolimus Immediate Release to LCP Tacrolimus in Obese Kidney Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/conversion-of-tacrolimus-immediate-release-to-lcp-tacrolimus-in-obese-kidney-transplant-recipients/. Accessed March 7, 2026.« Back to 2022 American Transplant Congress