Comparison of Pharmacokinetic and Clinical Outcomes of Tacrolimus Hexal® with Prograf® Based Regimen in De Novo Kidney Transplant Recipients: Results from the Randomized, SparTacus Study.
1SparTacus Study Group, Cologne, Germany
2Novartis Pharma GmbH, Nuernberg, Germany.
Meeting: 2016 American Transplant Congress
Abstract number: A164
Keywords: Calcineurin, Immunosuppression, Kidney transplantation, Pharmacokinetics
Session Information
Session Name: Poster Session A: Kidney: Acute Cellular Rejection
Session Type: Poster Session
Date: Saturday, June 11, 2016
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Halls C&D
Background: There is very limited evidence on the pharmacokinetic (PK) and clinical outcomes, comparing a generic tacrolimus with the standard reference drug in transplant (Tx) setting. The SparTacus (NCT01649427) study was designed to evaluate the PK profile and clinical data of tacrolimus Hexal® vs. Prograf® in de novo kidney Tx recipients (KTxR).
Methods: A total of 76 de novo KTxR were randomized in this prospective, two-phase open-label study to receive either tacrolimus Hexal® (n=35) or Prograf® (n=41), both in combination with enteric-coated mycophenolate sodium + corticosteroids + induction therapy with basiliximab. Starting dose of tacrolimus was 0.15 mg/kg/day, adjusted to target trough levels (C0) of 8–12 ng/mL from Tx to month (M) 1; 5–10 ng/mL up to M3; and 5–8 ng/mL up to M6. Primary objective (phase I) was to demonstrate comparable PK of tacrolimus Hexal® vs Prograf® as assessed by the ratio of the AUC0-12h over a period of 1-M post-Tx. Here we present the PK results at M1 along with efficacy and safety data at M6.
Results: Of 76 patients, 44 patients had an evaluable PK profile on Day 3, 10 and M1 and were included in PK analysis (tacrolimus Hexal®, n=23; Prograf®, n=21). A total of 73 patients were included in safety analysis (tacrolimus Hexal®, n=35; Prograf®, n=38). At M1, PK parameters: dose-normalized tacrolimus 12-h-AUC (h/103xL), Cmax (1/103xL), and mean 12-h tacrolimus C0 ([mu]g/L) were comparable between the treatment arms. At M6, tacrolimus Hexal® vs Prograf® had comparable incidence of composite events and its individual components (biopsy-proven acute rejection, graft loss, or death). Incidence of adverse events (AEs) and serious AEs were comparable between the treatment groups.
Conclusion: Tacrolimus Hexal® and Prograf® in de novo KTxR had a similar PK profile with comparable efficacy and safety. 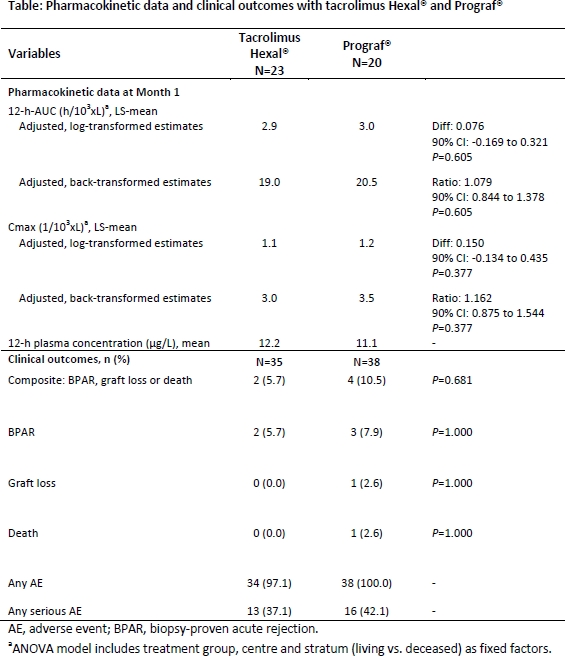
CITATION INFORMATION: Arns W, Huppertz A, Rath T, Ziefle S, Rump L, Hansen A, Baeumer D, Budde K, Lehner L, Klein T, Schenker P. Comparison of Pharmacokinetic and Clinical Outcomes of Tacrolimus Hexal® with Prograf® Based Regimen in De Novo Kidney Transplant Recipients: Results from the Randomized, SparTacus Study. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Arns W, Huppertz A, Rath T, Ziefle S, Rump L, Hansen A, Baeumer D, Budde K, Lehner L, Klein T, Schenker P. Comparison of Pharmacokinetic and Clinical Outcomes of Tacrolimus Hexal® with Prograf® Based Regimen in De Novo Kidney Transplant Recipients: Results from the Randomized, SparTacus Study. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/comparison-of-pharmacokinetic-and-clinical-outcomes-of-tacrolimus-hexal-with-prograf-based-regimen-in-de-novo-kidney-transplant-recipients-results-from-the-randomized-spartacus-study/. Accessed July 1, 2025.« Back to 2016 American Transplant Congress
