Comparing The Immunologic Responses Between Kidney Transplant Recipients Receiving Standard Immunosuppression And CNI Reduction After Two Doses Of ChAdOx-1 And A Booster Dose BNT162b2
1Nephrology division, Department of medicine, King Chulalongkorn Memorial Hospital, Bangkok, Thailand, 2King Chulalongkorn Memorial Hospital, Bangkok, Thailand, 3Bamrasnaradura infectious disease institute, Bangkok, Thailand, 4Infectious diseases, Department of medicine, King Chulalongkorn Memorial Hospital, Bangkok, Thailand, 5Department of Microbiology, King Chulalongkorn Memorial Hospital, Bangkok, Thailand, 6Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Bangkok, Thailand
Meeting: 2022 American Transplant Congress
Abstract number: 9010
Keywords: COVID-19, Immunosuppression, Kidney transplantation, Vaccination
Topic: Basic & Clinical Science » Basic & Clinical Science » 73 - COVID-19
Session Information
Session Name: Late Breaking: COVID-19
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 2:00pm-3:00pm
 Presentation Time: 2:30pm-2:40pm
Presentation Time: 2:30pm-2:40pm
Location: Hynes Room 310
*Purpose: Immunosuppression can impair immunological responses after vaccination in kidney transplant recipients (KTR). However, little is known about the effect of the booster dose of COVID-19 vaccination, with BNT162b2, after two doses of ChAdOx-1 and its response especially comparing among KTR with different immunosuppressive regimen.
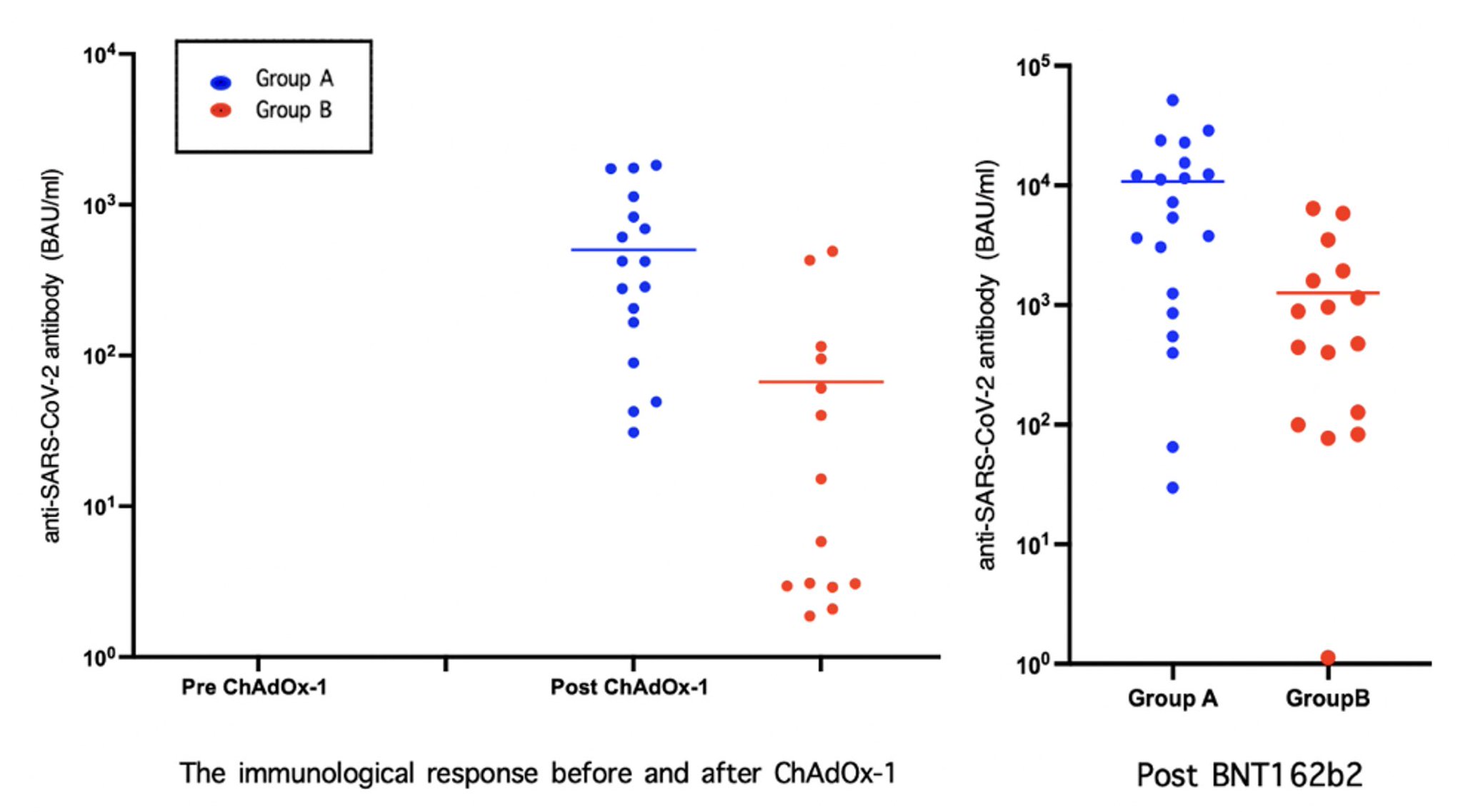
*Methods: KTRs who had stable allograft function with the regimen of calcineurin inhibitor (CNI) reduction (sirolimus, tacrolimus [Ctrough 2-4 ng/mL], prednisolone; group A) and the standard regimen (tacrolimus [Ctrough 4-7 ng/mL], mycophenolate mofetil, prednisolone; group B) were enrolled for two doses of ChAdOx-1 (three months apart) and a booster dose with BNT162b2 (1 month after ChAdOx-1). KTRs who had history of COVID-19, anti-SARS-CoV-2 antibody positive at baseline, and positive for donor specific antibody (DSA) were excluded. The immune response for vaccination was measured by anti-SARS-CoV-2 antibody one month after two doses of ChAdOx-1 and BNT162b2. DSA (Luminex®) was measured at one month after BNT162b2.
*Results: Forty KTR were enrolled with mean age of 47.52 ± 10.86 years old. There were 21 KTR in group A and 19 KTR in group B. The group A has significantly higher anti-SARS-CoV-2 antibody after 2 doses of ChAdOx-1 (502.05 ± 612.87 BAU/ml and 66.69 ±143.01 BAU/ml for group A and B, p < 0.05) and after a booster dose of BNT162b2 (10,291.04 ± 12,803.95 BAU/ml and 1,264.94 ±1,935.50 BAU/ml for group A and B, p < 0.05). None of KTRs developed DSA after completing all vaccination including the booster dose.
*Conclusions: The KTR with CNI reduction regimen has significantly higher immunologic response to COVID-19 vaccination. The booster dose of COVID-19 vaccination provides significantly improve in immunological response in both standard and CNI reduction regimens. Two doses of ChAdOx-1 and a booster dose of BNT162b2 did not lead to DSA production in both regimens.
To cite this abstract in AMA style:
Phirom S, Kitrungphaiboon T, Jantarabenjakul W, Avihingsanon Y, Prasithsirikul W, Vanichanan J, Hansasuta P, Townamchai N. Comparing The Immunologic Responses Between Kidney Transplant Recipients Receiving Standard Immunosuppression And CNI Reduction After Two Doses Of ChAdOx-1 And A Booster Dose BNT162b2 [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/comparing-the-immunologic-responses-between-kidney-transplant-recipients-receiving-standard-immunosuppression-and-cni-reduction-after-two-doses-of-chadox-1-and-a-booster-dose-bnt162b2/. Accessed July 3, 2025.« Back to 2022 American Transplant Congress