Combination of Non-HLA and HLA-DSA Antibodies Identifies Liver Transplants Recipients With Highest Risk for Fibrosis Progression and Mortality
1Simmons Transplant Institute, Baylor University Medical Center, Dallas, TX
2Terasaki Foundation Laboratory, Los Angeles, CA
3Celltrend, Berlin, Germany
4Nephrology & Intensive Care Medicine, Charite Berlin, Berlin, Germany.
Meeting: 2015 American Transplant Congress
Abstract number: 391
Keywords: Alloantibodies, HLA antibodies, Liver transplantation
Session Information
Session Name: Concurrent Session: Liver: Immunosuppression and Rejection
Session Type: Concurrent Session
Date: Tuesday, May 5, 2015
Session Time: 2:15pm-3:45pm
 Presentation Time: 2:15pm-2:27pm
Presentation Time: 2:15pm-2:27pm
Location: Room 120-ABC
Recent data has shown an increased risk of fibrosis progression in HCV viremic liver transplant (LT) recipients with HLA DSA in serum. However, the role of non-HLA DSA and the interaction between HLA and non-HLA DSA remains unknown in LT patients. Methods: 535 HCV-viremic primary liver allograft recipients at Baylor University Medical Center between 1/00 to 4/09 had their prospectively collected pre-transplant serum tested retrospectively for the following alloantibodies: Class I & II HLA DSA (MFI>5000), Angiotensin II Type-1 Receptor (AT1R) DSA (>19 U/mL), and Endothelin-1 Type A receptor (ETAR) DSA (>23 U/mL). Results: Preformed DSA were found in the following patients: Class I &/or II HLA DSA alone (69), AT1R (76), ETAR (64), HLA DSA with AT1R (13), HLA DSA with ETAR (12). Fibrosis progression to the composite endpoint of METAVIR stage 2-4 or death caused by liver allograft failure was markedly accelerated in patients with the combination of a preformed HLA and non-HLA DSA compared to patients with either none or 1 DSA (Figure 1; p=0.01). Similar findings were seen when each HLA DSA was combined with AT1R (p=0.04) or ETAR (p=0.04). Stepwise multivariable analysis, controlling for donor and recipient race, recipient age, CMV infection, sustained virologic response, and induction therapy, showed the combination of a preformed non-HLA and HLA-DSA had a HR=2.33 (p=0.01) of advanced fibrosis or liver related death. Conclusions: The combination of a preformed non-HLA (either AT1R or ETAR) and HLA DSA was associated in multivariable modeling with the highest hazard ratio for METAVIR stage 2-4 or liver related death of any other single variable.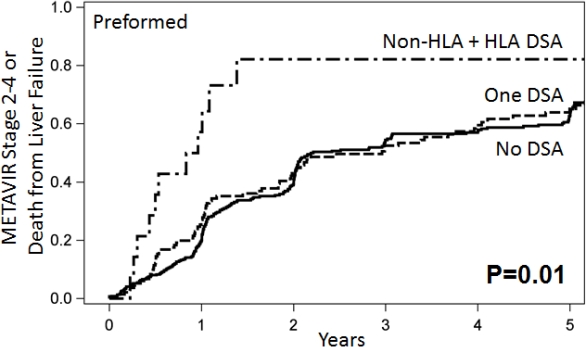
To cite this abstract in AMA style:
O'Leary J, Cai J, Heidecke H, Jennings L, Philippe A, Harbour V, Catar R, Terasaki P, Klintmalm G, Dragun D. Combination of Non-HLA and HLA-DSA Antibodies Identifies Liver Transplants Recipients With Highest Risk for Fibrosis Progression and Mortality [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/combination-of-non-hla-and-hla-dsa-antibodies-identifies-liver-transplants-recipients-with-highest-risk-for-fibrosis-progression-and-mortality/. Accessed March 2, 2026.« Back to 2015 American Transplant Congress
