Clinical Validation of Automated Urinary Chemokine Assays for Non-Invasive Detection of Kidney Transplant Rejection: A Large Prospective Cohort Study
1Microbiology, Immunology and Transplantation, KU Leuven, Leuven, Belgium, 2ESAT STADIUS Center for Dynamical Systems, KU Leuven, Leuven, Belgium, 3Department of Nephrology and Kidney Transplantation, Necker Hospital, Paris, France
Meeting: 2022 American Transplant Congress
Abstract number: 466
Keywords: Kidney transplantation, Monitoring, Non-invasive diagnosis, Rejection
Topic: Basic Science » Basic Clinical Science » 17 - Biomarkers: Clinical Outcomes
Session Information
Session Name: Biomarkers: Clinical Outcomes I
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 4:00pm-4:10pm
Presentation Time: 4:00pm-4:10pm
Location: Hynes Veterans Auditorium
*Purpose: Non-invasive diagnostic markers for kidney transplant rejection can provide useful information but often do not reach the clinical implementation stage. The chemokines CXCL9 and CXCL10 have been extensively studied as biomarkers for rejection. However, final validation in a sufficiently large, real-world prospective cohort is needed to determine the relation to rejection subtypes and clinical confounders, the context of use, and the added benefit for clinical practice.
*Methods: In this single-center prospective cohort study, we analyzed 1559 biopsy-paired urinary samples at both indication and protocol time points from 622 kidney transplants performed between April 2013 and July 2019. We quantified urinary CXCL9 and CXCL10 using automated immuno-assays and normalized the values to urinary creatinine. Next to evaluating the diagnostic performance for Banff-grade rejection, we also considered rejection as assessed by the molecular microscope.
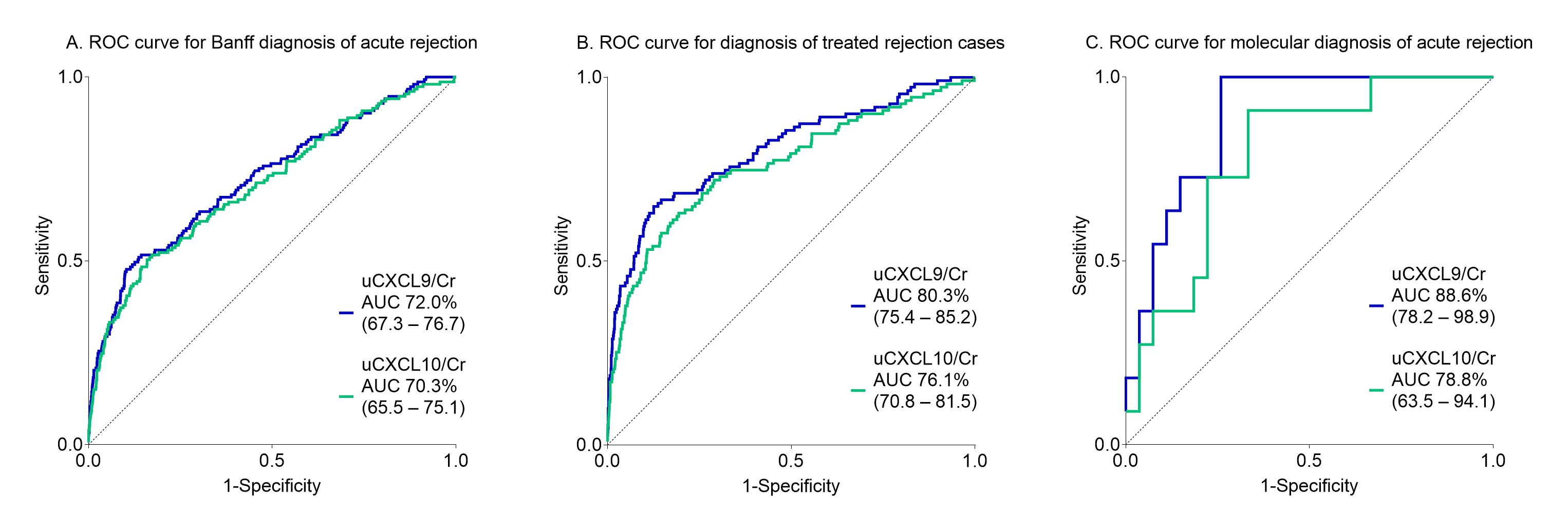
*Results: The urinary chemokines strongly associated with the biopsy’s inflammation severity, when considering classical semi-quantitative histological scoring, but also when considering molecular rejection scores. The diagnostic performance of urinary chemokines alone was moderate for Banff acute rejection, and increased when considering treated rejections or molecular rejection instead (Figure 1). Taking into account confounding factors rather than excluding them, an integrated model of urinary chemokines with eGFR, donor-specific antibodies, time after transplantation and polyoma viremia had high diagnostic value for acute rejection (N=150) (ROCAUC 82.6%, 95% CI 78.9-86.2) and added value on top of clinical standard-of-care. The integrated score would help to safely avoid 59 protocol biopsies per 100 patients when the predicted rejection risk is below 10%.
*Conclusions: Integrating urinary chemokines and clinical markers allows for personalized non-invasive monitoring of rejection probability and enables to importantly and safely reduce the number of unnecessary biopsies. We anticipate this study paves the way to final implementation of urinary chemokine measurements as non-invasive markers for rejection in routine clinical follow-up after kidney transplantation.
To cite this abstract in AMA style:
Loon EVan, Tinel C, Callemeyn J, Coemans M, Craenenbroeck AVan, Vaulet T, Anglicheau D, Naesens M. Clinical Validation of Automated Urinary Chemokine Assays for Non-Invasive Detection of Kidney Transplant Rejection: A Large Prospective Cohort Study [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/clinical-validation-of-automated-urinary-chemokine-assays-for-non-invasive-detection-of-kidney-transplant-rejection-a-large-prospective-cohort-study/. Accessed July 5, 2025.« Back to 2022 American Transplant Congress