Clinical Outcomes with IR-tac to LCP-tac Conversion in a Kidney Transplant Population
F. Bartlett1, J. Newman1, T. Sprague1, S. Patel1, N. Rao2, E. Andrade2, V. Rohan2, M. Casey2, D. Dubay1, D. Taber1
1Pharmacy, Medical University of South Carolina, Charleston, SC, 2Medical University of South Carolina, Charleston, SC
Meeting: 2022 American Transplant Congress
Abstract number: 994
Keywords: Adverse effects, Graft function, Infection, Kidney transplantation
Topic: Clinical Science » Infection Disease » 25 - Kidney Infectious Non-Polyoma & Non-Viral Hepatitis
Session Information
Session Name: Kidney Infectious Non-Polyoma & Non-Viral Hepatitis
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: LCP-Tac is approved for conversion from IR-Tac in kidney transplant patients with a conversion rate of 70-80%. Studies have demonstrated similar efficacy and safety outcomes between the two agents, with improvement in neurotoxicity with LCP-Tac. The goal of this study was to assess real world long-term safety and effectiveness outcomes after conversion from IR-Tac to LCP-Tac in a large kidney transplant population.
*Methods: This was a retrospective longitudinal cohort study of adult kidney transplant recipients converted from IR-Tac to LCP-Tac between June 2019 and October 2020. Patients converted back to IR-Tac within 1 month were excluded. Tacrolimus pharmacokinetic data and clinical outcomes were collected 1-year pre- and post-conversion. Continuous data were analyzed using a paired T-test and categorical data using the McNemar test.
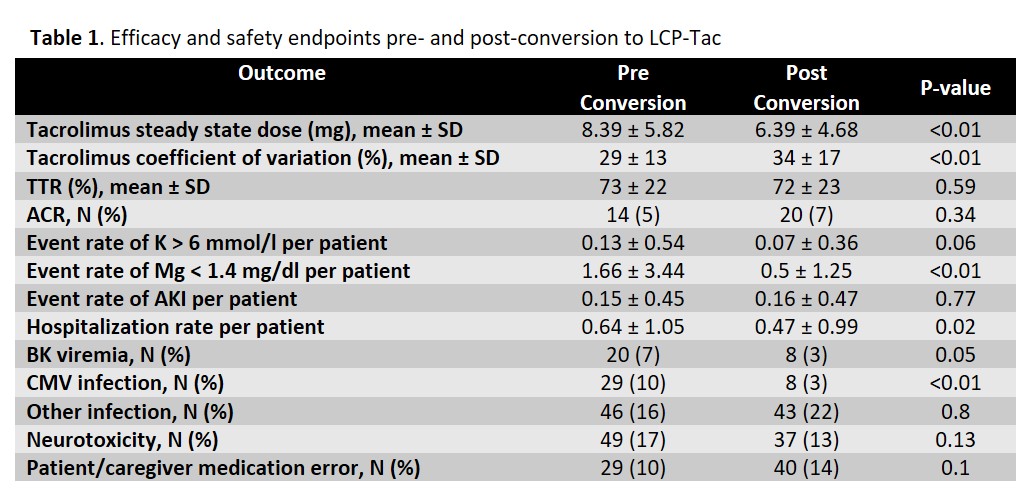
*Results: A total of 337 patients were screened with 292 patients included in the analysis. The mean recipient age was 52.9 years; 63% were black and 43% were female. Median number of days from transplant to conversion was 481 (227,775) with 35% converted within 1-year. There was no difference in acute cellular rejection rates pre- and post-conversion (ACR; P=0.34), with similar time in therapeutic range (TTR; P=0.59) as well. Coefficient of variation was higher post-conversion (29 v 34%, P<0.01). There was a higher rate of CMV infection pre-conversion (10 v 3%, P<0.01) and numerically higher rate of BK viremia (7 v 3%, P=0.05). The rate of other documented infections was similar pre- and post-conversion. Neurotoxicity burden tended to being less common post-conversion, as did hypomagnesemia and hyperkalemia (Table 1). Graft and patient survival were excellent (95% and 97%, respectively) at 3-years post-transplant.
*Conclusions: Conversion to LCP-Tac in kidney transplantation is safe, with similar rates of infection, AKI, and rejection pre- and post-conversion. Neurotoxicity burden and electrolyte abnormalities tended to be less common after conversion to LCP-Tac.
To cite this abstract in AMA style:
Bartlett F, Newman J, Sprague T, Patel S, Rao N, Andrade E, Rohan V, Casey M, Dubay D, Taber D. Clinical Outcomes with IR-tac to LCP-tac Conversion in a Kidney Transplant Population [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/clinical-outcomes-with-ir-tac-to-lcp-tac-conversion-in-a-kidney-transplant-population/. Accessed March 9, 2026.« Back to 2022 American Transplant Congress