Clinical Outcomes from a Mobile Health, Pharmacist-Led Intervention: 12-Month Results of the Transafe Rx Randomized Controlled Trial
Med Univ of South Carolina, Charleston, SC
Meeting: 2020 American Transplant Congress
Abstract number: LB-2
Keywords: Graft failure, Graft function, Immunosuppression, Kidney transplantation
Session Information
Session Name: Late Breaking Oral Abstract
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:27pm-3:39pm
Presentation Time: 3:27pm-3:39pm
Location: Virtual
*Purpose: Medication errors and non-adherence are predominant causes of poor graft outcomes in kidney transplant (KTX) recipients. The primary aim of the TRANSAFE Rx RCT was to assess the efficacy of a pharmacist-led, mHealth-based intervention at improving medication safety and clinical outcomes, as compared to usual care in KTX.
*Methods: This was a 12-month, parallel arm, 1:1 RCT in adult KTX 6 to 36 months post-transplant (NCT03247322). All received usual post-transplant care, while those randomized to the intervention arm received clinical pharmacist-led supplemental med therapy monitoring and management via a smartphone-enabled mHealth application, bioinformatic web portal, risk-based televisits, and home-based BP and glucose monitoring. The primary clinical aim was to assess healthcare utilization, graft function, acute rejection rates, infection rates, and graft and patient survival between arms at 12-months post-randomization in an intent-to-treat semi-blinded analysis.
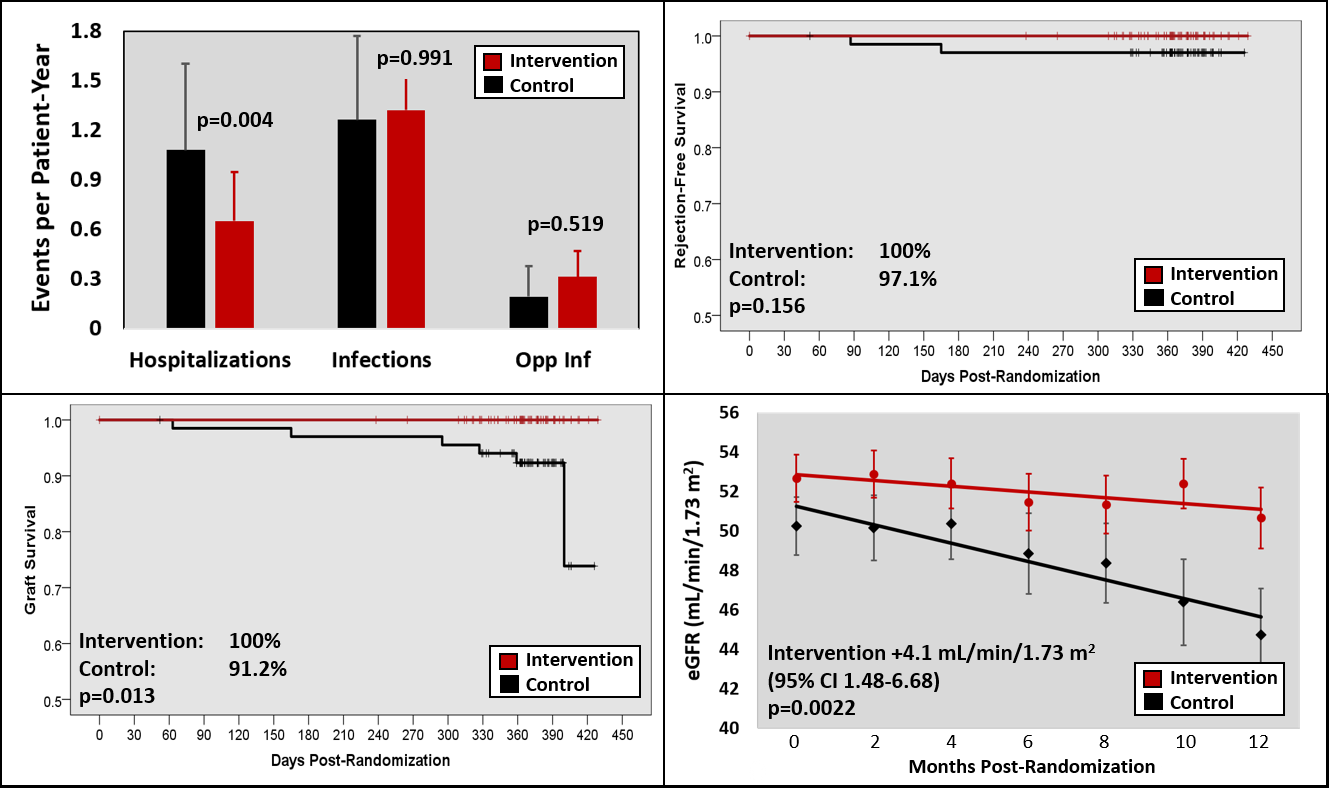
*Results: 136 patients were enrolled (68 in each arm) at a mean of 1.2±0.8 years post-transplant; 2 patients withdrew for a 98.5% retention rate. Baseline characteristics were similar between arms, except more DM in the control arm and more CMV D+/R- in the intervention arm. Over the 12-month follow-up, the intervention arm had significantly lower rates of hospitalization (1.1 vs. 0.65 hospitalizations per patient-year; adjusted IRR 0.46 95% CI 0.27-0.77; p=0.0036), with similar rates of clinic visits, procedures, and infections (top left panel in Figure). Over the 12-month study, there were 3 biopsy proven and treated rejections in 2 participants in the control arm and none in the intervention arm (two Banff 1B and one AMR, p=0.156; top right panel in Figure); there was one death in the control arm (ovarian CA) and no deaths in the intervention arm (p=0.237). Graft survival was 100% in the intervention arm and 91.2% in the control arm (p=0.013, bottom left panel in Figure). Two graft losses were due to acute rejection (Banff 1B and AMR), two were due to progressive decline in graft function, one was from borderline rejection in a marginal graft, and one was due to death. GFR slope was significantly better in the intervention arm (+4.1 mL/min/1.73m2 over the 12-month study, p=0.0022; bottom right panel in Figure).
*Conclusions: A pharmacist-led mHealth intervention demonstrated improved outcomes over a 12-month study period, including significantly lower rates of hospitalization and better graft survival, when compared to usual care.
To cite this abstract in AMA style:
Taber D, Hirsch J, Su Z, McGillicuddy J, Posadas A, Fleming J. Clinical Outcomes from a Mobile Health, Pharmacist-Led Intervention: 12-Month Results of the Transafe Rx Randomized Controlled Trial [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/clinical-outcomes-from-a-mobile-health-pharmacist-led-intervention-12-month-results-of-the-transafe-rx-randomized-controlled-trial/. Accessed February 19, 2026.« Back to 2020 American Transplant Congress