Clinical Outcome of Patients With De Novo C1q Binding Donor-Specific HLA Antibodies After Renal Transplantation
1Nephrology, Charite Hospital-University of Medicine, Berlin, Germany
2HLA Laboratory, Charite Hospital-University of Medicine, Berlin, Germany.
Meeting: 2015 American Transplant Congress
Abstract number: A291
Keywords: HLA antibodies, Kidney transplantation, Rejection
Session Information
Session Name: Poster Session A: Late Breaking
Session Type: Poster Session
Date: Saturday, May 2, 2015
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Exhibit Hall E
A. Not all de novo donor-specific HLA antibodies (dnDSA) are causing immediate graft loss. The importance of complement-binding DSA was recently shown with a commercial C1q Luminex assay. We developed a modified C1q-Luminex assay (Lachmann N et al, Transplantation 2013;95:694-700.) showing a higher sensitivity as compared to the CDC assay and hypothesize that, with this assay, we could differentiate between harmful (ABMR) or harmless dnDSA (stable clinical course).
B. We selected 59 renal transplant recipients (RTR) with dnDSA, available serum samples and sufficient follow-up after 1st dnDSA detection. Samples were both tested with screening Luminex and single antigen assays for dnDSA and with our modified assay for the presence of C1q-binding IgG dnDSA (C1q+).
C. 37 (63%) developed ABMR (mean follow-up 37±28 mths). 22 (37%) had no clinical/biological signs of graft dysfunction (mean follow-up 34±17 mths). Groups were comparable for baseline and donor characteristics. ABMR RTR had a significant higher frequency of class I (59% vs. 23%) or class I+II dnDSA (51% vs. 9%) and more pregnancies (88% vs. 17%) (p<0.01). Significantly more ABMR RTR had C1q+. The mean C1q+ MFI was significantly higher in the ABMR group (table1).
| dnDSA+ ABMR (n=37) | dnDSA+ no ABMR (N=22) | P* | |
| C1q+ dnDSA classe I and/or II n. (%) | 27(73) | 9(41) | 0.014 |
| Mean MFI C1q+ dnDSA positivity (±SD) | 5482±4498 | 2291±3348 | 0.005 |
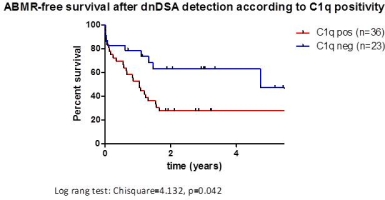 The sensitivity and specificity of the test were 73% and 59% with a PPV and NPV of 75% and 57%, respectively.
The sensitivity and specificity of the test were 73% and 59% with a PPV and NPV of 75% and 57%, respectively.
D. We demonstrate the clinical relevance of C1q-binding dnDSA on clinical allograft outcome. Not all RTR C1q+ have a poor outcome, specificity was only moderate. Yet, the assay was shown to be a useful tool to stratify the immunological risk for ABMR after detection of dnDSA.
To cite this abstract in AMA style:
Bamoulid J, Roodenburg A, Staeck O, Schoenemann C, Lachmann N, Budde K. Clinical Outcome of Patients With De Novo C1q Binding Donor-Specific HLA Antibodies After Renal Transplantation [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/clinical-outcome-of-patients-with-de-novo-c1q-binding-donor-specific-hla-antibodies-after-renal-transplantation/. Accessed June 30, 2025.« Back to 2015 American Transplant Congress
