Clazakizumab® (anti-il-6) Desensitization in Highly-HLA Sensitized Patients Awaiting Kidney Transplant (nct03380962): Long-Term Follow Up
1Comprehensive Transplant Center, Cedars Sinai Medical Ctr, Los Angeles, CA, 2HLA Laboratory, Cedars Sinai Medical Ctr, Los Angeles, CA, 3Pathology, Cedars Sinai Medical Ctr, Los Angeles, CA
Meeting: 2022 American Transplant Congress
Abstract number: 401
Keywords: B cells, Highly-sensitized, HLA antibodies, Kidney transplantation
Topic: Clinical Science » Kidney » 36 - Kidney Immunosuppression: Desensitization
Session Information
Session Name: Kidney Immunosuppression: Desensitization & Acute Antibody Mediated Rejection
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 3:40pm-3:50pm
Presentation Time: 3:40pm-3:50pm
Location: Hynes Ballroom A
*Purpose: IL-6 is a cytokine critical for B-cell activation and IgG production. Clazakizumab (CSL Behring LLC) is a humanized monoclonal aimed at the cytokine IL-6. Here we report on 24M follow-up of a single-center Phase I/II study of Clazakizumab for desensitization (DES) in highly-HLA sensitized (HS) patients.
*Methods: Twenty HS patients received PLEX x5 followed by IVIg 2gm/kg X1; then clazakizumab 25mg SC Q4W X6M w. monitoring of HLA antibody. Study end points examined reduction in HLA MFI, rates of transplantation and DSAs pre-& post-tx. Transplanted patients received clazakizumab 25mg Q4W x12M post-tx, induction with alemtuzumab, and maintained on tac/mmf/pred and protocol biopsy @6M.
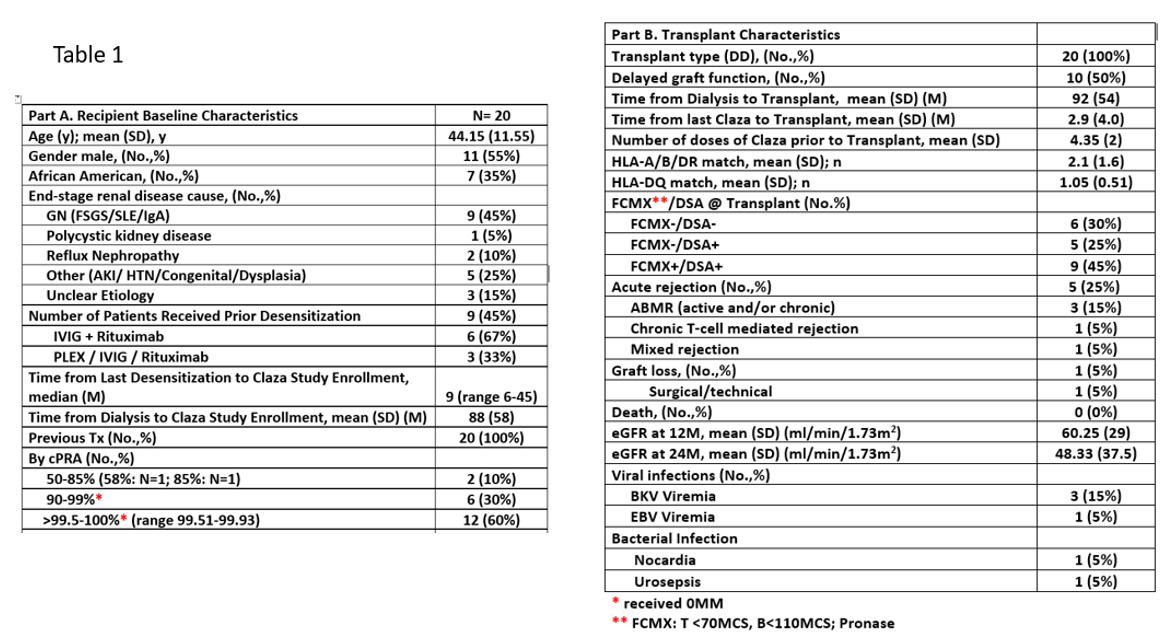
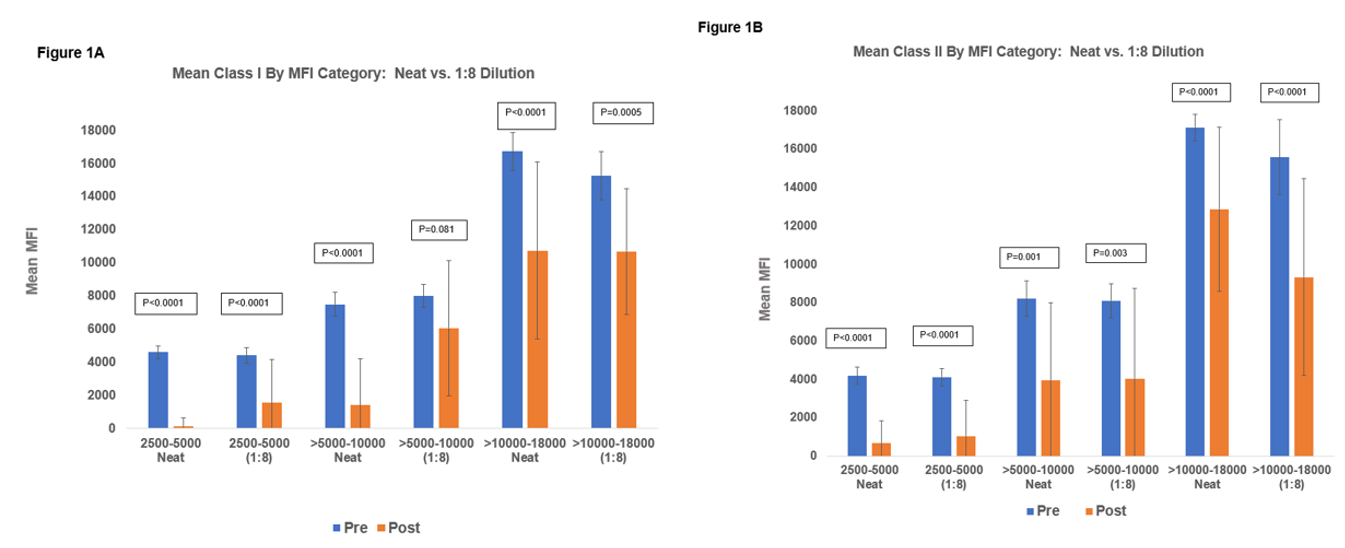
*Results: 20 HS patients were enrolled. All 20 received DDKT (18 (on-study) and 2 required further DES. Time from dialysis to transplant was 92±54M and from last clazakizumab to transplant was 2.9±4.0M, mean # of clazakizumab doses was 4.4±2.0. All patients had previous transplants; 60% had cPRA>99.95%, 65% were FCMX+/DSA+ @transplant (Table 1). Figure 1A & 1B show the effect of clazakizumab on HLA class I and class II, by strength of MFI pre-DES and 6M post-clazakizumab at neat & 1:8 dilution. Impact on MFIs were: ≥10000: pre- vs. post-DES, class I: 16,742±1137 vs. 10,671±5346 (at neat; p<0.001) and 15,295±1463 vs. 10,709±3802 (1:8 dilution; p=0.005); class II: 17,136±702 vs. 12,879±4282 (at neat; p=0.001) and 15,589±1949 vs. 9337±5136 (1:8 dilution; p<0.001), respectively. AMR episodes occurred in 4 patients with low Banff 2019 scores. Importantly, no DSA rebound was seen in the first-year post-tx. Mean eGFR @24M was 48±38ml/min/1.73m2.
*Conclusions: Clazakizumab treatment was associated with significant reductions in HLA alloantibodies and DSAs, increased transplant rates for highly-sensitized patients and low rejection rates. However, confirmation of efficacy for desensitization requires assessment in randomized controlled trials.
To cite this abstract in AMA style:
Vo A, Tang J, Ammerman N, Huang E, Zhang X, Haas M, Peng A, Najjar R, Williamson S, Meyers C, Sethi S, Lim K, Gillespie M, Badash N, Jordan S. Clazakizumab® (anti-il-6) Desensitization in Highly-HLA Sensitized Patients Awaiting Kidney Transplant (nct03380962): Long-Term Follow Up [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/clazakizumab-anti-il-6-desensitization-in-highly-hla-sensitized-patients-awaiting-kidney-transplant-nct03380962-long-term-follow-up/. Accessed March 7, 2026.« Back to 2022 American Transplant Congress