CFZ533: Assessment of Immunomodulatory Activity Following Single Doses of a Novel Anti-CD40 mAb in Healthy Volunteers
1Novartis Institute for Biomedical Research, East Hanover, NJ
2Novartis Institute for Biomedical Research, Basel, Switzerland
3Celerion, Inc, Lincoln, NE.
Meeting: 2015 American Transplant Congress
Abstract number: 307
Keywords: B cells, Co-stimulation, Immunosuppression, Vaccination
Session Information
Session Name: Concurrent Session: Kidney: Novel Agents
Session Type: Concurrent Session
Date: Monday, May 4, 2015
Session Time: 4:00pm-5:30pm
 Presentation Time: 4:36pm-4:48pm
Presentation Time: 4:36pm-4:48pm
Location: Terrace I-III
Blocking the CD40-CD154 pathway has been shown to effectively prolong renal allograft survival in non-human primates. CFZ533 is a novel, fully human, Fc-silent, anti-CD40 monoclonal antibody being developed for use in transplantation. In non-clinical studies, CFZ533 prolonged allograft survival and inhibited T-dependent antibody response (TDAR). To provide an early assessment of the immunosuppressive activity in humans, an immune challenge sub-study was included in the first-in-human trial in healthy subjects. METHODS: A double-blind, placebo controlled, ascending, single-dose study with IV infusions of 0.03, 0.1, 0.3, 1.0 and 3.0 mg/kg or 3.0 mg/kg CFZ533 subcutaneously was conducted. All subjects were immunized with a single subcutaneous dose of a neo-antigen, Keyhole Limpet Hemocyanin (KLH) with alum adjuvant on days 3 and 85. Blood samples for PK, CD40 receptor occupancy (RO) and anti-KLH IgG and IgM profiling were collected at multiple time points during the study. Anti-KLH antibodies were assessed in a validated human ELISA system with a LOQ of 0.7 (IgG) and 2.1 (IgM) μg/mL. RESULTS: A total of 48 subjects were enrolled. All doses of CFZ533 and KLH were well tolerated. CFZ533 PK concentrations were quantifiable at all dose levels tested. At the highest CFZ533 dose, 3 mg/kg, complete peripheral CD40 RO was maintained for 28 days. Representative time-KLH response curves following a single 3 mg/kg dose of CFZ533 are shown in Figure 1.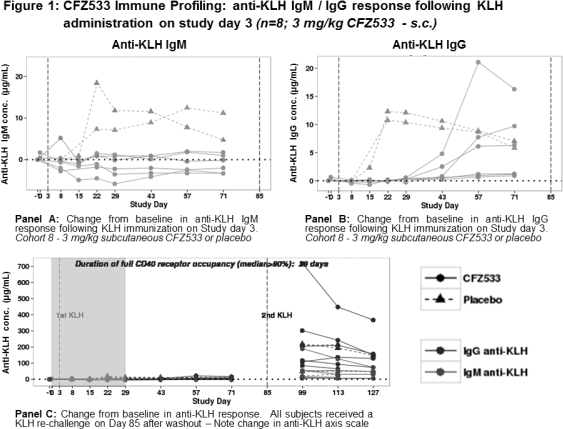 Over the 28 days of full peripheral CD40 RO, complete suppression of the primary immune response was evident in all CFZ533-treated subjects (Panel A/B). After complete CFZ533 washout, all subjects mounted a robust anti-KLH response following a KLH re-challenge on day 85 (Panel C). CONCLUSION: The favorable safety and tolerability profile of CFZ533 coupled with a predictable concentration-CD40 RO relationship and suppression of a primary T-dependent antibody response supports future clinical trials of CFZ533 in transplantation.
Over the 28 days of full peripheral CD40 RO, complete suppression of the primary immune response was evident in all CFZ533-treated subjects (Panel A/B). After complete CFZ533 washout, all subjects mounted a robust anti-KLH response following a KLH re-challenge on day 85 (Panel C). CONCLUSION: The favorable safety and tolerability profile of CFZ533 coupled with a predictable concentration-CD40 RO relationship and suppression of a primary T-dependent antibody response supports future clinical trials of CFZ533 in transplantation.
To cite this abstract in AMA style:
Slade A, Doucet J, Koo P, Espie P, Rush J, Tomek C, Klupp J. CFZ533: Assessment of Immunomodulatory Activity Following Single Doses of a Novel Anti-CD40 mAb in Healthy Volunteers [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/cfz533-assessment-of-immunomodulatory-activity-following-single-doses-of-a-novel-anti-cd40-mab-in-healthy-volunteers/. Accessed March 9, 2026.« Back to 2015 American Transplant Congress
