Building a Third-Party VST Bank from Scratch- The Cincinnati Experience
A. S. Nelson,1 D. Heyenbruch,2 X. Zhu,3 S. M. Davies,1 C. Lutzko,3 C. Bollard,4 P. Hanley,4 T. Leemhuis,2 L. Danziger-Isakov,5 M. S. Grimley.1
1Bone Marrow Transplant & Immune Deficiency, Cincinnati Children's Hospital Medical Center, Cincinnati, OH
2Hoxworth Blood Center, Univeristy of Cincinnati, Cincinnati, OH
3Experimental Hematology & Cancer Biology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH
4Proram for Cell Enhancement and Technologies for Immunotherapy, Children's National Health System, Washington, DC
5Depatment of Infectious Diseases, Cincinnati Children's Hospital Medical Center, Cincinnati, OH.
Meeting: 2018 American Transplant Congress
Abstract number: D285
Keywords: Bone marrow transplantation, Infection, Pediatric, Viral therapy
Session Information
Session Name: Poster Session D: Late Breaking
Session Type: Poster Session
Date: Tuesday, June 5, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Background
Viral infections remain a challenge to treat and significantly contribute to morbidity and mortality. Virus specific T cells (VSTs) have shown clinical efficacy, with minimal toxicity and long term efficacy. We sought to actively build a third-party VST bank, for use in eligible patients.
Methods
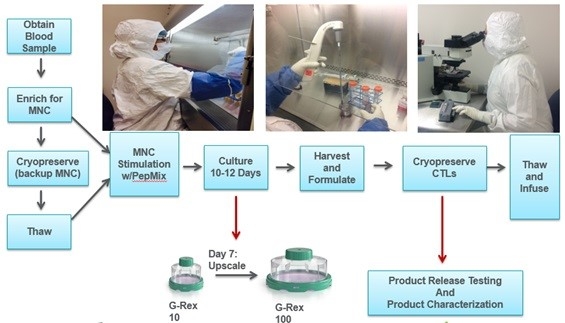
VSTs targeting CMV, adenovirus and EBV were manufactured using APC pulsed with commercially available peptide pools (PepMixes) to expand EBV/CMV/Ad specific T cells (Figure 1).
Results
A total of 30 products are currently in the third-party VST bank ready for use.
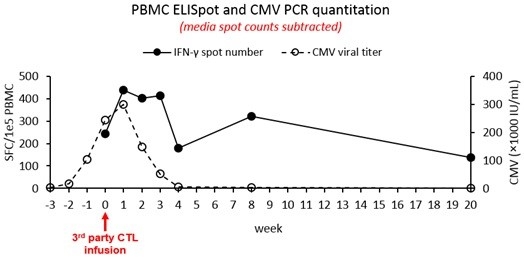
Thirteen VST infusions have been given to 7 patients. Eleven infusions have been given for CMV and two for adenovirus. Five out of seven patients responded to third-party VST infusions (Figure 2). The median HLA matching was 2 out of 10 per patient (range 1 to 4)
No patients experienced adverse reactions or GVHD
Conclusion
A third-party VST bank is feasible and produces clinically appropriate VSTs for use in patients with viral infections. HLA typing and matching of VST products is essential to reduce toxicity and promote appropriate antigen presentation and expansion of VSTs in vivo. Further work is underway to further characterize the VSTs to better define the HLA restriction and immunogenicity of each VST product.
CITATION INFORMATION: Nelson A. S., Heyenbruch D., Zhu X., Davies S. M., Lutzko C., Bollard C., Hanley P., Leemhuis T., Danziger-Isakov L., Grimley M. S. Building a Third-Party VST Bank from Scratch- The Cincinnati Experience Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Nelson AS, Heyenbruch D, Zhu X, Davies SM, Lutzko C, Bollard C, Hanley P, Leemhuis T, Danziger-Isakov L, Grimley MS. Building a Third-Party VST Bank from Scratch- The Cincinnati Experience [abstract]. https://atcmeetingabstracts.com/abstract/building-a-third-party-vst-bank-from-scratch-the-cincinnati-experience/. Accessed February 26, 2026.« Back to 2018 American Transplant Congress