Brincidofovir for Treatment of Disseminated Human Adenovirus Infection in Pediatric Solid Organ Transplant Recipients
J. Londeree1, R. Garro1, R. Romero2, R. George1, P. Winterberg1, R. Liverman3, A. Serluco3, S. Shin1, I. Yildirim4
1Pediatric Nephrology, Emory, Atlanta, GA, 2Pediatric Hepatology and Gastroenterology, Emory, Atlanta, GA, 3Pharmacy, Children's Healthcare of Atlanta, Atlanta, GA, 4Pediatric Infectious Disease, Emory, Atlanta, GA
Meeting: 2020 American Transplant Congress
Abstract number: C-182
Keywords: Adenoviruses, Kidney transplantation, Liver transplantation, Pediatric
Session Information
Session Name: Poster Session C: All Infections (Excluding Kidney & Viral Hepatitis)
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Brincidofovir (BCV) is an investigational antiviral drug with potency against Human Adenovirus (HAdV). We describe four cases of disseminated HAdV infection treated with (BCV) among pediatric solid organ transplant (SOT) patients.
*Methods: We retrospectively reviewed 4 cases of severe disseminated HAdV infection, which occurred between 2018 and 2019, in a tertiary pediatric transplant center. HAdV infection was defined as a positive HAdV PCR from a clinical specimen. Baseline clinical characteristics, disease course, treatment, and outcomes were reviewed.
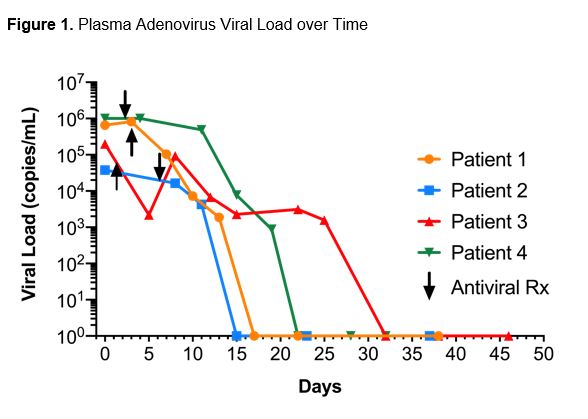
*Results: Four pediatric SOT patients (2 kidney, 1 dual kidney/liver and 1 liver) ranging in age from 9 months to 19 years were diagnosed with HAdV infection (Table 1). Patients presented with varied symptoms from 12 to 912 days post-transplant. Peak HAdV viral load ranged from 37,000 to >1,000,000 copies/mL. All patients had disseminated disease with evidence of graft involvement and varying degrees of acute kidney injury (AKI) (Table 2) making them candidates for BCV therapy over cidofovir to avoid nephrotoxicity. Three out of four patients received cidofovir prior to conversion to BCV. IRB approved compassionate use of BCV was dosed at 2 mg/kg (max 100 mg) twice weekly PO along with reduced immunosuppression. Resolution of symptoms and HAdV viremia was seen at a mean of 21.5 days (range 15-32) of therapy. All patients had resolution of AKI with a return to baseline creatinine after therapy.
*Conclusions: Though HAdV infection in pediatric SOT patients is uncommon, a subset of patients experience severe disease complicated by graft dysfunction. Although supportive care and decreased immunosuppression remain the cornerstone of therapy, BCV was well tolerated and efficacious in our series of patients. Further research is needed to better understand risk factors for HAdV infection and treatment options to improve patient outcomes.
To cite this abstract in AMA style:
Londeree J, Garro R, Romero R, George R, Winterberg P, Liverman R, Serluco A, Shin S, Yildirim I. Brincidofovir for Treatment of Disseminated Human Adenovirus Infection in Pediatric Solid Organ Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/brincidofovir-for-treatment-of-disseminated-human-adenovirus-infection-in-pediatric-solid-organ-transplant-recipients/. Accessed July 14, 2025.« Back to 2020 American Transplant Congress