Bone Marrow Resident Long-Lived Plasma Cells as Source of Chronic Donor-Specific Antibodies
Dept. of General Surgery, Div. of Transplantation, Medical University of Vienna, Vienna, Austria
Meeting: 2022 American Transplant Congress
Abstract number: 1221
Keywords: B cells, Bone marrow, HLA antibodies
Topic: Basic Science » Basic Science » 04 - B-cell / Antibody /Autoimmunity
Session Information
Session Name: B-cell / Antibody /Autoimmunity
Session Type: Poster Abstract
Date: Monday, June 6, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: The importance of bone marrow resident plasma cells for long-lasting humoral immunity has been highlighted in several models for vaccination and infectious diseases. To which extent this cell population is also responsible for upholding a sustained humoral response against (donor)-HLA antigens in the transplant setting remains unclear. We therefore sought to identify and characterize DSA-secreting plasma cells to provide insight into their underlying biology.
*Methods: C57BL/6 were grafted with a fully mismatched balb/c cardiac allograft without any immunosuppressive treatment. Serum DSA were assessed regularly via flow crossmatch. Spleen and bone marrow cells of each cardiac allograft recipient were harvested 20 weeks post transplantation und cultured separately for 48h. DSA within the cell culture supernatants were measured via flow crossmatch. Splenic and bone marrow plasma cells were quantified and characterized using flow cytometry in transplant recipients and age-matched naïve individuals. To directly identify DSA-secreting plasma cells, fluorophore conjugated donor MHC-tetramers were used for intracellular staining of fixed and permeabilized plasma cells.
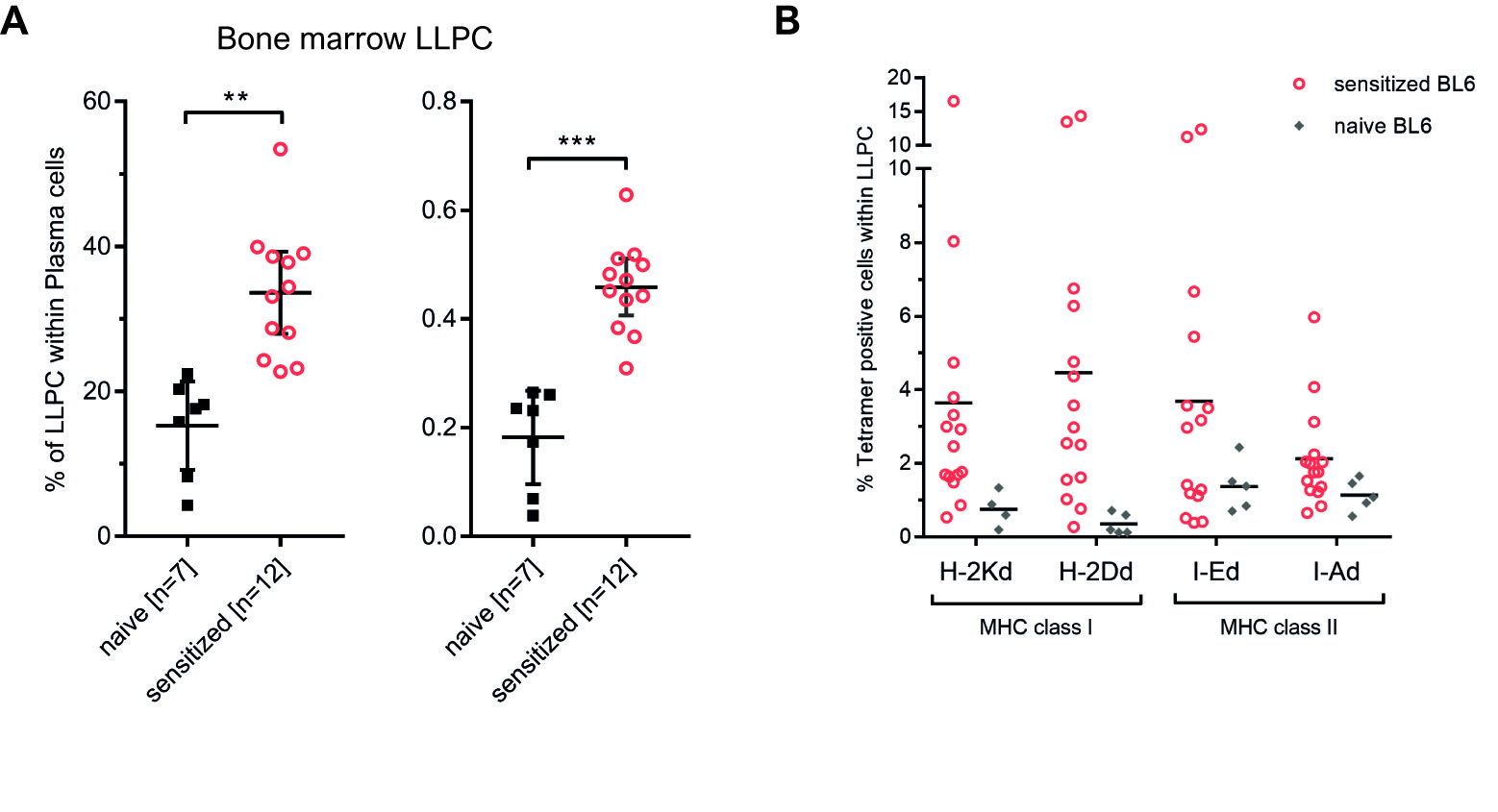
*Results: Fully mismatched balb/c heart allografts were rapidly rejected within 12 days in untreated BL6 recipients and elicited a substantial humoral allo-immune response. Bone marrow and spleen cells harvested from each individual cardiac allograft recipient 20 weeks post transplantation were cultured separately. A flow crossmatch of the supernatant demonstrated that this chronic DSA response was almost exclusively carried by the bone marrow rather than the spleen (mean in-vitro IgG DSA MFI; bone marrow: 441 vs. spleen: 59; p=0.0002). Within their bone marrow, transplant recipients carried a substantially greater proportion and absolute number of long-lived plasma cells (LLPC; B220- CD138+ TACI+ CD19-) (% LLPC within all plasma cells; naïve (n=7): 15.26 +/- 4.88% vs. HTX recipients (n=12): 33.60 +/- 5.04%; p=0.002) (LLPC x106; naïve (n=7): 0.18 +/- 0.07 vs. HTX recipients (n=12): 0.46 +/- 0.04; p=0.0004) [Fig. 1A]. In-situ staining of DSA within permeabilized plasma cells using fluorophore conjugated donor-MHC tetramers demonstrated that a substantial proportion of bone-marrow LLPCs secretes donor-MHC specific IgG [Fig. 1B].
*Conclusions: Bone marrow resident long-lived plasma cells represent a crucial source of late donor-specific antibodies.
To cite this abstract in AMA style:
Muckenhuber M, Pichler S, Weijler AM, Wekerle T. Bone Marrow Resident Long-Lived Plasma Cells as Source of Chronic Donor-Specific Antibodies [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/bone-marrow-resident-long-lived-plasma-cells-as-source-of-chronic-donor-specific-antibodies/. Accessed February 25, 2026.« Back to 2022 American Transplant Congress