Biomarkers in Multivisceral and Intestinal Transplantation
1Pediatric Gastroenterology, University of Miami, Miami, FL, 2Surgery, Division of Liver/GI Transplant, University of Miami, Miami, FL, 3Public Health Sciences, Division of Biostatistics, University of Miami, Miami, FL, 4Pediatric Infectious Diseases and Immunology, University of Miami, Miami, FL
Meeting: 2019 American Transplant Congress
Abstract number: D349
Keywords: Graft failure, Infection, Rejection, Renal injury
Session Information
Session Name: Poster Session D: Small Bowel: All Topics
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: Multivisceral transplant (MVT) and isolated intestinal transplant (ITx) are complex surgical procedures that result in a pro-inflammatory state, making interpretation of biomarkers in the immediate postoperative (post-op) period difficult. The objective of this study was to establish the trend of various biomarkers in the immediate post-op period in MVT and ITx and to evaluate their use as diagnostic markers of complications.
*Methods: This single center prospective study was approved by our university’s Institutional Review Board. Recipients of new MVT/ITx of all ages at our institution were eligible for inclusion. Recipients of MVT/ITx prior to the onset of the study period, who were re-transplanted during the study period, were excluded. Data was collected for the first 4 weeks post-op and included: recipient and donor demographics, type of transplant and indication, clinical status at time of transplant, ischemia and operating times, and complications (infection, acute kidney injury, rejection, and graft failure). Biomarkers assessed include white cell count (WBC), neutrophil lymphocyte count ratio (NLCR), immature granulocyte %, eosinophil %, immature platelet fraction (IPF), and procalcitonin. Biomarkers were collected preoperatively, post-op days 1, 3, 5, and 7, and weekly until the end of the 4 week period. For statistical analysis, times that didn’t have at least 2 recipients with valid data at each time were eliminated.
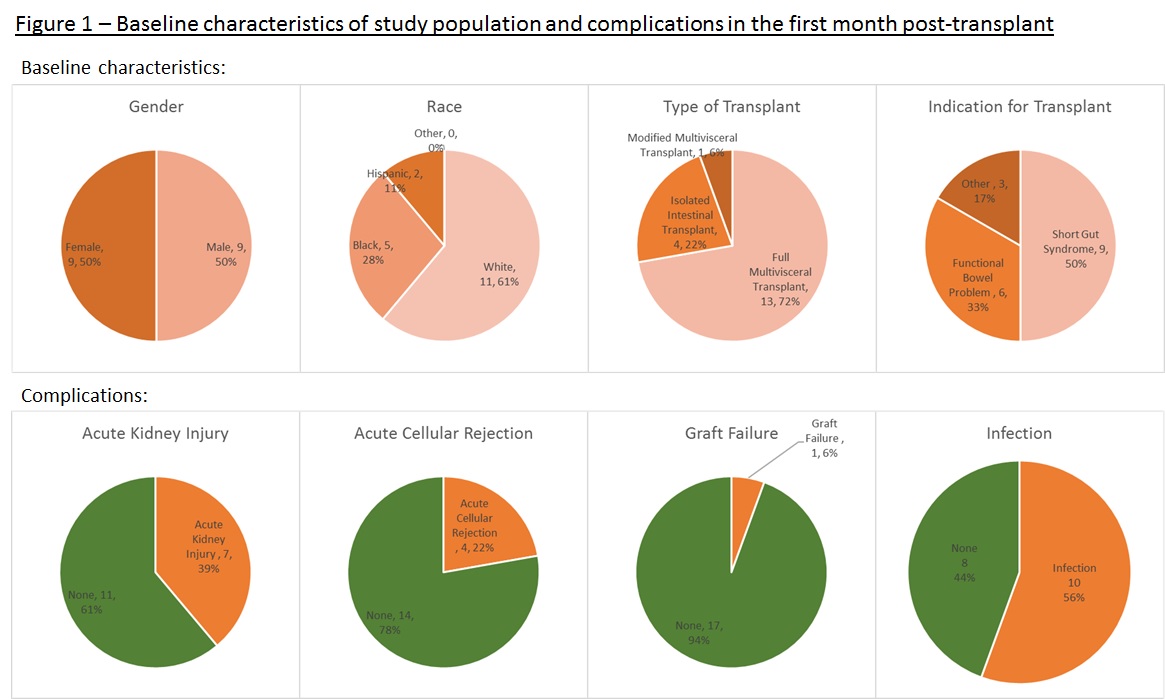
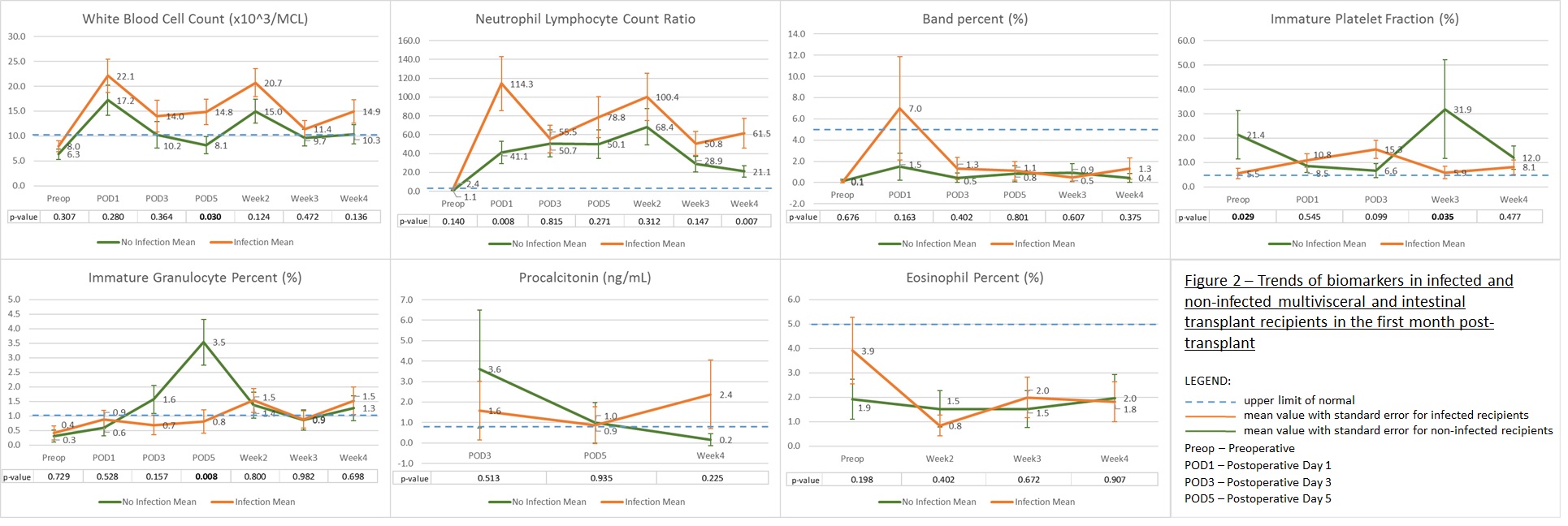
*Results: Over a 16 month period (July 2017-Oct 2018), twenty-seven subjects were enrolled of whom 18 received their MVT/ITx. Recipients were aged 2 to 67-years with a median age of 25-years. The male:female and the pediatric:adult recipient ratio were equally distributed. See Fig. 1 for baseline characteristics and complications. Ten recipients (56%) had a clinically significant infection requiring antimicrobials which occurred on post-op days 3 to 28 with a median day on day 14. Donor, recipient and surgical data collected were not found to be useful for predicting an infection. The trends of biomarkers in infected and non-infected recipients in the first month post-transplant are displayed in Fig. 2. NLCR and IPF post-transplant are higher than established upper limits of normal, regardless of infection status. WBC and NLCR were on average higher in infected compared to non-infected recipients but was only statistically significant (p-value <0.05) on 1 and 2 time points out of 7, respectively.
*Conclusions: This is the first study to extensively trend biomarkers post-MVT/ITx. Multi-center studies are needed to provide sufficient power to establish normal ranges in these recipients and to determine if WBC and NLCR are the best biomarkers to diagnose an infection.
To cite this abstract in AMA style:
Cheung DA, Garcia J, Beduschi T, Langshaw AH, Arheart KL, Gonzalez IA. Biomarkers in Multivisceral and Intestinal Transplantation [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/biomarkers-in-multivisceral-and-intestinal-transplantation/. Accessed February 22, 2026.« Back to 2019 American Transplant Congress