Belatacept Conversion in Proteinuric Kidney Transplant Recipients: Effects in a Prospective Trial and a Retrospective Cohort
1Center for Transplantation Sciences, Massachusetts General Hospital, Charlestown, MA, 2UT Southwestern Medical Center, Dallas, TX, 3Brigham and Women's Hospital, Boston, MA
Meeting: 2022 American Transplant Congress
Abstract number: 303
Keywords: Glomerular filtration rate (GFR), Graft function, Immunosuppression, Proteinuria
Topic: Clinical Science » Kidney » 46 - Kidney Complications: Non-Immune Mediated Late Graft Failure
Session Information
Session Name: Kidney Complications: Non-Immune Mediated Late Graft Failure
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 6, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:30pm-6:40pm
Presentation Time: 6:30pm-6:40pm
Location: Hynes Room 310
*Purpose: Proteinuria after kidney transplantation is associated with worse allograft outcomes. B7-1 expression by podocytes has been linked to proteinuria by inducing podocyte migration which can be targeted by belatacept. We examined the anti-proteinuric effects of calcineurin inhibitor (CNI) to belatacept conversion in proteinuric kidney transplant recipients (KTRs).
*Methods: In a phase I single-arm multicenter prospective trial, we recruited EBV IgG+ adult KTRs >6 months post-kidney transplantation with an eGFR >30 ml/min/1.73m2, proteinuria >1 g/day, and CNI-based immunosuppression. Patients were converted from CNI to belatacept. The primary outcome was 25% reduction in proteinuria at 12 months. In addition, we collected retrospective data from KTRs >3 months post-KT with proteinuria >0.4 g/day who were converted from CNI to belatacept at MGH. For comparison, we also included a retrospective control cohort of CNI patients with 0.4 g/day of proteinuria who remained on CNI.
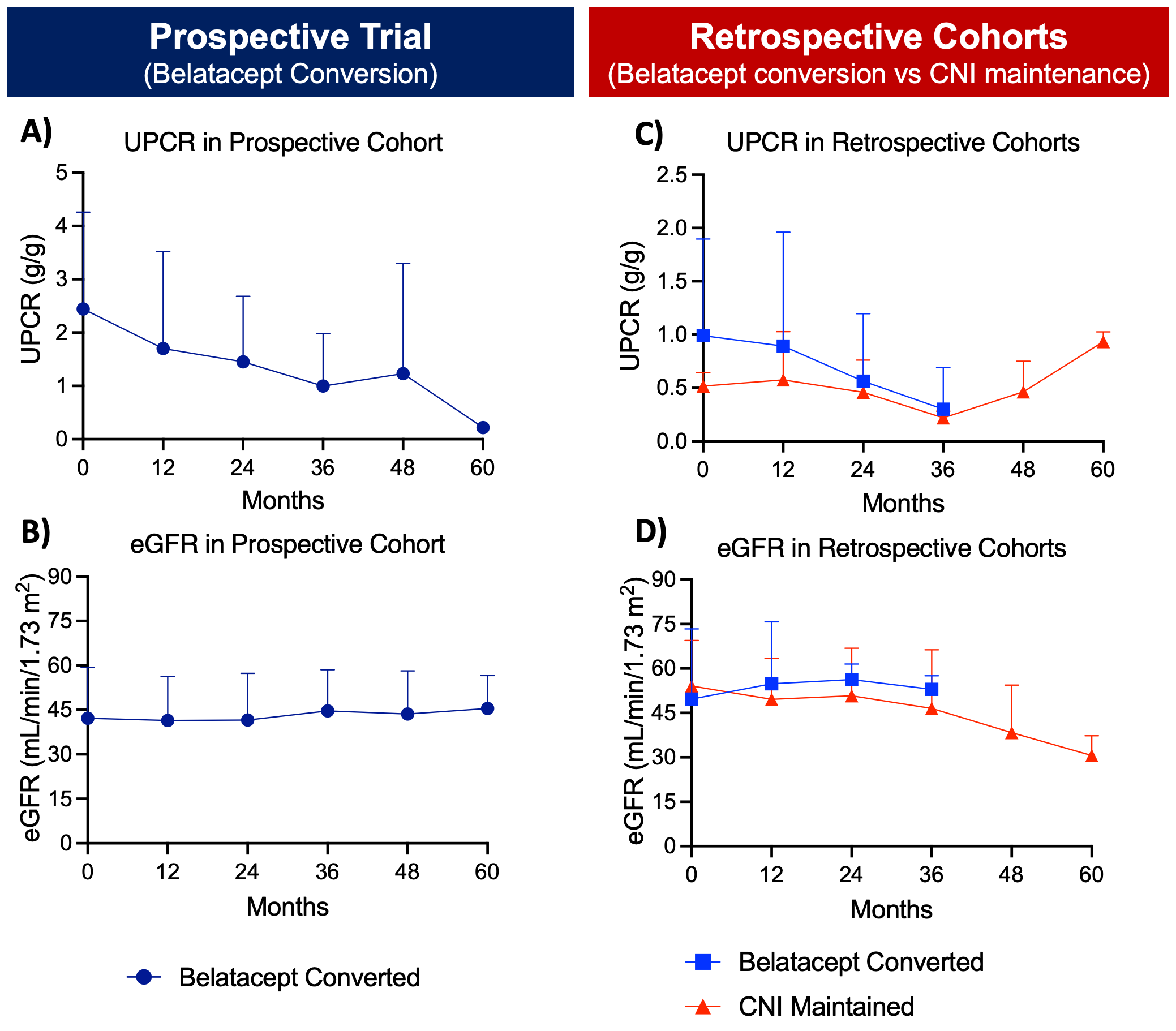
*Results: In the prospective cohort, 15 KTRs were recruited who had pre-conversion mean eGFR (+SD) of 45.7+13 and median (IQR) proteinuria of 1.8 (1.4-3.5) g/g. At 12 months post-conversion, mean eGFR remained stable at 43.7+13 ml/min/1.73m2 and the primary outcome of 25% reduction in proteinuria was reached in 53% of KTRs with median overall reduction to 1.4 (0.45-2.25) g/g. None of the patients had graft rejection in the first year. One patient had worsening proteinuria and discontinued belatacept. In the retrospective cohort, 9 of 77 belatacept conversion patients had pre-conversion proteinuria >0.4 g/g and available follow-up values. Baseline and post-conversion 12-months median proteinuria were 0.5 (0.47-1.53) g/g and 0.19 (0.12-1.06) g/g, respectively (p=0.496). Mean eGFRs was 49.7+24 at baseline and it remained stable at 54.9+21 ml/min/1.73m2 at 12 months (p=0.24). In the CNI-maintained control cohort (n=21), baseline and 12-month mean proteinuria were 0.52+0.13 and 0.55+0.42 g/g and mean eGFRs dropped from 54+15 to 50+14 ml/min/1.73m2, respectively. Figure 1 summarizes the proteinuria and eGFR values from 3 cohorts.
*Conclusions: Belatacept conversion in proteinuric KTRs leads to stable allograft function and a slight reduction in proteinuria at one year and beyond.
To cite this abstract in AMA style:
Efe O, Jurdi AAl, Wojciechowski D, Safa K, Gilligan H, Chandraker A, Azzi J, Weins A, Riella LV. Belatacept Conversion in Proteinuric Kidney Transplant Recipients: Effects in a Prospective Trial and a Retrospective Cohort [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/belatacept-conversion-in-proteinuric-kidney-transplant-recipients-effects-in-a-prospective-trial-and-a-retrospective-cohort/. Accessed July 3, 2025.« Back to 2022 American Transplant Congress