Belatacept and Tacrolimus Corticosteroid-Free Regimens Lead to Elevated Mycophenolic Acid Exposure at 1 and 3 Months after Kidney Transplantation
UCincinnati, Cincinnati.
Meeting: 2018 American Transplant Congress
Abstract number: C91
Keywords: Adverse effects, Area-under-curve (AUC), Immunosuppression, Mycophenolate mofetil
Session Information
Session Name: Poster Session C: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Monday, June 4, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Mycophenolate mofetil (MMF) is generally administered as a fixed dose. Studies have shown a relationship between MPA exposure (MPAAUC) but no data evaluates this relationship in CNI/steroid-free regimens. Also, the relationship between MPAAUC and adverse effects (AEs) is not well-established. Our objective was to evaluate the incidence of adverse events per MPAAUC in a prospective trial.
Method: Prospective MPAAUC measurement protocol in renal transplants (RT) receiving CNI or belatacept-based steroid-free regimens with rATG or alemtuzumab induction at 1 (MPAAUCM1) and 3 (MPAAUCM3) months post RT using the Pawinski equation (7.75+6.49C0+0.76C0.5+2.43C2). MPAAUC ranges were: low (< 30mg/L*h-1), intermediate (30-60mg/L*h-1) and high (AUC >60mg/L*h-1). MMF dose was adjusted per MPAAUC, AEs (viremia, leukopenia, neutropenia, BPAR1yr) and provider discretion. An AE was considered associated with an MPAAUC when it occured within 1 month of the measurement.
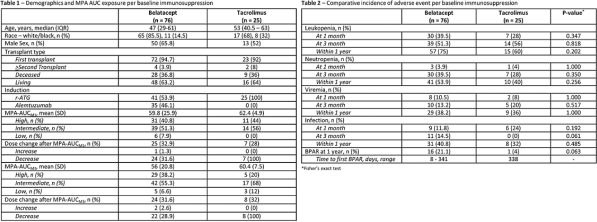
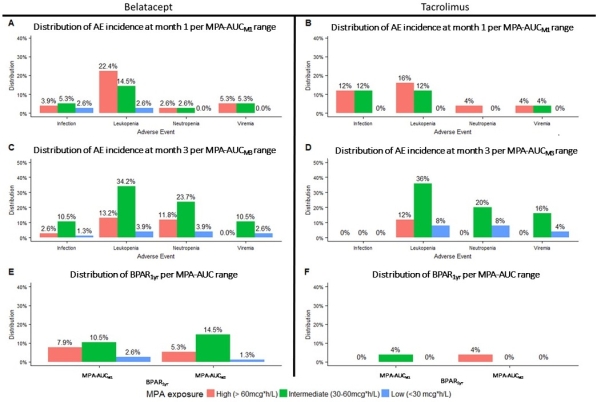
Results: 101 patients were analyzed. Demographics, MPAAUCs, and AEs per IS group are included in Tables 1 and 2. Mean MPAAUCs were elevated (range 56-62.4mg/L*h-1). Following MMF dosage decrease at month 1 in >30% of patients, mean MPAAUCM3 remains similar to MPAAUCM1. Figure 2 (panels A-D) represents the distribution of overall AEs at 1 and 3 months per MPAAUC groups and panels E-F the distribution of BPAR1yr.
Conclusion: Regardless of concomitant IS, MPAAUCM1 was high in 1/3 of patients; low MPAAUC was rare. Despite similar MPAAUCM1, leukopenia occurred more frequently in belatacept vs tacrolimus regimens. Similar MPAAUCM3 following dose decrease at 1 month indicates potential increase in MPA exposure over time post RT. As no relationship between MPAAUC and AEs has been observed, studies to determine optimal MPAAUC should be conducted.
CITATION INFORMATION: Dao A., Tremblay S., Shields A., Loethen A., Abu Jawdeh B., Diwan T., Govil A., Woodle E., Alloway R. Belatacept and Tacrolimus Corticosteroid-Free Regimens Lead to Elevated Mycophenolic Acid Exposure at 1 and 3 Months after Kidney Transplantation Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Dao A, Tremblay S, Shields A, Loethen A, Jawdeh BAbu, Diwan T, Govil A, Woodle E, Alloway R. Belatacept and Tacrolimus Corticosteroid-Free Regimens Lead to Elevated Mycophenolic Acid Exposure at 1 and 3 Months after Kidney Transplantation [abstract]. https://atcmeetingabstracts.com/abstract/belatacept-and-tacrolimus-corticosteroid-free-regimens-lead-to-elevated-mycophenolic-acid-exposure-at-1-and-3-months-after-kidney-transplantation/. Accessed February 20, 2026.« Back to 2018 American Transplant Congress