B-Cell Tolerance to Donor Alloantigens
Department of Surgery, Transplantation Biology Research Center, Massachusetts General Hospital, Boston, MA.
Meeting: 2015 American Transplant Congress
Abstract number: 516
Keywords: Alloantibodies, B cells, Tolerance
Session Information
Session Name: Concurrent Session: Late Breaking
Session Type: Concurrent Session
Date: Tuesday, May 5, 2015
Session Time: 2:15pm-3:45pm
 Presentation Time: 2:15pm-2:27pm
Presentation Time: 2:15pm-2:27pm
Location: Terrace IV
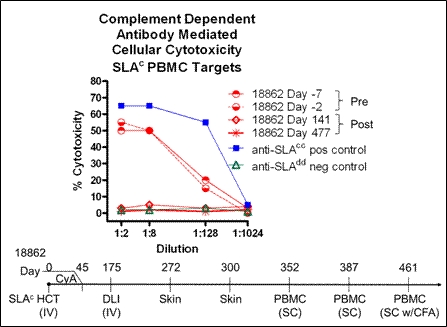
Pre-existing panel-reactive antibodies and development of donor-specific alloantibodies are known to play important roles in graft dysfunction and deterioration. Preventing and/or eliminating alloantibody responses would dramatically improve organ transplantation outcomes. In our large animal experience of hematopoietic cell transplantation (HCT) with reduced intensity conditioning, alloantibody responses are not detected even in swine recipients that do not engraft and eventually lose chimerism. Here we report that intravenous (IV) exposure to unmobilized donor peripheral blood cells under a novel reduced intensity conditioning protocol consisting of 100cGy total body irradiation, CD3-immunotoxin mediated T-cell depletion and a short course of Cyclosporin A (CyA) reliably results in stable donor-specific B-cell tolerance across swine leukocyte antigen (SLA) barriers in miniature swine. Transient T-cell hyporesponsiveness to donor cells is observed followed by regain of T-cell alloresponses after the loss of chimerism. B-cell unresponsiveness persists despite development of sensitized T-cell alloresponses following a donor leukocyte infusion (DLI), rejection of donor allografts, and multiple subcutaneous (SC) injections of donor cells. The humoral tolerance established is donor specific with preserved antibody responses to third party antigens as tested by immunization with vaccines, complete Freund's adjuvant (CFA) and keyhole limpet hemocyanin. Even animals with evidence for pre-existing cytotoxic antibodies to donor cells prior to transplantation are successfully tolerized at the B-cell level using this protocol (Figure 1). Recent data demonstrate that exposure to and rejection of a vascularized skin allograft during the period of transient donor cell chimerism under these mildly immunosuppressive conditions does not abrogate B-cell tolerance induction in this model. These results show that robust B-cell tolerance can be established through IV exposure to donor hematopoietic cells under certain mildly immunosuppressive conditions even in pre-sensitized recipients.
To cite this abstract in AMA style:
Patil A, Climov M, Matar A, Musa A, Harrington E, Sachs D, Duran-Struuck R, Huang C. B-Cell Tolerance to Donor Alloantigens [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/b-cell-tolerance-to-donor-alloantigens/. Accessed February 24, 2026.« Back to 2015 American Transplant Congress
