Assessment of Bleeding and Thrombosis in Responders and Initial Non-Responders to Aspirin After Continuous-Flow Left Ventricular Assist Device Placement
Medical University of South Carolina, Charleston, SC.
Meeting: 2015 American Transplant Congress
Abstract number: C176
Keywords: Dosage, Efficacy, Heart assist devices, Post-operative complications
Session Information
Session Name: Poster Session C: "Loss of Breath": VADs and Other Pre-Heart Transplant Matters
Session Type: Poster Session
Date: Monday, May 4, 2015
Session Time: 5:30pm-6:30pm
 Presentation Time: 5:30pm-6:30pm
Presentation Time: 5:30pm-6:30pm
Location: Exhibit Hall E
Purpose: This study evaluates bleeding and thrombosis following left ventricular assist device (LVAD) placement using platelet inhibition monitoring to determine responders to standard aspirin dosing (81mg) compared to inital non-responders who were titrated upward to achieve therapeutic response.
Methods: Patients ≥18 years undergoing LVAD implant from 1/09–7/14 initially received standard aspirin dosing (81mg) post-opt. Responsiveness to initial aspirin therapy was assessed by the VerifyNow® Aspirin Test and defined as <550 aspirin reaction units (ARU). In non-responders (≥550ARU), aspirin doses were uptitrated and retested to verify responsiveness. Outcomes between the groups were evaluated and subgroup analysis of initial aspirin responders (<500ARU vs 500–549ARU) was also performed.
Results: Response to initial aspirin dose was seen in 71.6% of patients. There were no significant differences in thrombosis or bleeding events between responders and inital non-responders to aspirin. INR did not differ significantly up to 12 months between the cohorts. Overall survival, freedom from thrombosis, and freedom from bleeding at 1 year are 79±9%, 89±4% and 63±6% and at 2 years are 79±9%, 89±4% and 47±8% respectively. Comparing initial responders vs non-responders, there were no differences in overall survival (p=0.130), thrombosis(p=0.215), or bleeding (p=0.541) at 6 months. No significant differences in thrombosis (<500 ARU, n=37: 0.013/pt. mo, 95% CL:0.006 – 0.0247; 500 – 549 ARU, n=16: 0.023/pt. mo., 95% CL: 0.0074 – 0.053) or bleeding events (<500 ARU: 0.033/pt. mo, 95% CL: 0.0211 – 0.0499; 500 – 549 ARU: 0.077/pt. mo., 95% CL: 0.045 – 0.1237) were detected between initial responders. 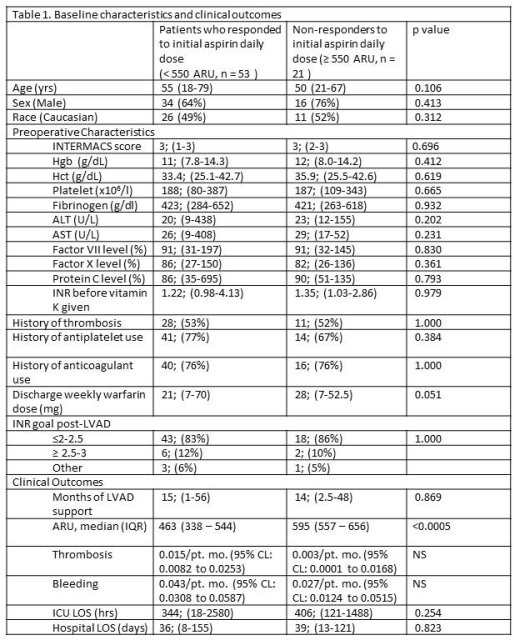
Conclusion: Non-responsiveness to standard aspirin doses was detected in several patients. Increasing aspirin therapy to achieve response in inital non-responders was associated with similar outcomes. There was no association between degree of platelet inhibition and event rates. Individualized treatment with platelet function testing may reduce complications.
To cite this abstract in AMA style:
Floroff C, Rieger K, Veasey T, Strout S, Meadows H, Stroud M, Toole J, Brisco M, Cook J, Lazarchick J, Uber W. Assessment of Bleeding and Thrombosis in Responders and Initial Non-Responders to Aspirin After Continuous-Flow Left Ventricular Assist Device Placement [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/assessment-of-bleeding-and-thrombosis-in-responders-and-initial-non-responders-to-aspirin-after-continuous-flow-left-ventricular-assist-device-placement/. Accessed February 21, 2026.« Back to 2015 American Transplant Congress
