Artificial Intelligence Based Dosing of Tacrolimus in Liver Transplantation: Prospective, Randomized Phase 2 Trial
A. Zarrinpar1, C. Ho2, C. Warren1, J. Khong2, M. Lee2, S. Duarte1, K. Andreoni1, M. Johnson1, N. Battula3, D. McKimmy1, T. Beduschi1
1Surgery, University of Florida, Gainesville, FL, 2Mechanical and Aerospace Engineering, University of California, Los Angeles, CA, 3Surgery, University of Oklahoma, Oklahoma City, OK
Meeting: 2022 American Transplant Congress
Abstract number: 540
Keywords: Dosage, Immunosuppression, Liver transplantation
Topic: Clinical Science » Organ Inclusive » 72 - Machine Learning, Artificial Intelligence and Social Media in Transplantation
Session Information
Session Name: Machine Learning, Artificial Intelligence and Social Media in Transplantation
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:20pm-6:30pm
Presentation Time: 6:20pm-6:30pm
Location: Hynes Room 210
*Purpose: Inter- and intra-individual variability in calcineurin dose requirements necessitates empirical clinician-titrated dosing that frequently results in deviation from target ranges, particularly during the critical early post-transplant period. We have developed an artificial intelligence-based approach, Phenotypic Personalized Medicine (PPM), to individualize drug dosing.
*Methods: In this single-center, randomized, partially blinded trial, participants were assigned immediately prior to liver transplantation 1:1 to standard-of-care clinician-guided dosing or PPM-guided dosing. Blood tacrolimus trough levels were measured daily until patient discharge. The primary outcome measure was fraction of days with large (> 2 ng/ml) deviations from target range. Secondary outcomes included post-transplant length of stay, fraction of days outside-of-target-range, mean area-under-the-curve outside-of-target-range, graft rejection, graft failure, death, infections, nephrotoxicity, or neurotoxicity. Patients were followed until discharge from hospital.
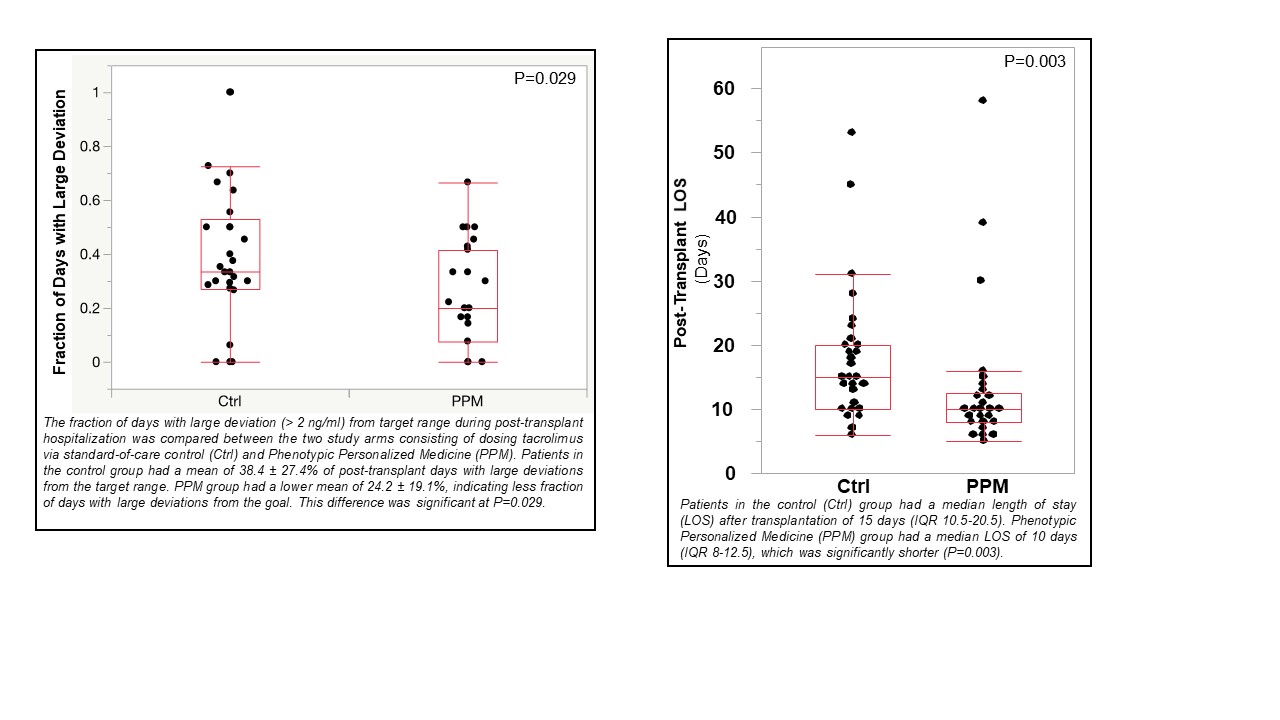
*Results: Sixty-two patients were screened and randomized. 31 were assigned to the control group and 31 to the PPM group. Fraction of post-transplant days with large deviation from target range was higher in the standard-of-care group than in the PPM group (38.4 ± 27.4% versus 24.2 ± 19.1%; P=0.03). Median length of stay was longer in the standard-of-care group than the PPM group [15 days (IQR 10.5-20.5) versus 10 days (IQR 8-12); P=0.003]. There were no significant differences in fraction of days outside-of-target-range, mean area-under-the-curve outside-of-target-range, graft rejection, graft failure, death, infections, nephrotoxicity, or neurotoxicity. No tacrolimus dosing related adverse events occurred during the trial.
*Conclusions: In this randomized prospective clinical trial of AI-based personalized dosing of tacrolimus in patients after liver transplantation, PPM-guided dosing resulted in a lower fraction of inpatient days where the tacrolimus trough blood levels had a large deviation from the target range. PPM patients also had 33% shorter post-transplant length of stay than patients receiving standard-of-care tacrolimus dosing.
To cite this abstract in AMA style:
Zarrinpar A, Ho C, Warren C, Khong J, Lee M, Duarte S, Andreoni K, Johnson M, Battula N, McKimmy D, Beduschi T. Artificial Intelligence Based Dosing of Tacrolimus in Liver Transplantation: Prospective, Randomized Phase 2 Trial [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/artificial-intelligence-based-dosing-of-tacrolimus-in-liver-transplantation-prospective-randomized-phase-2-trial/. Accessed March 9, 2026.« Back to 2022 American Transplant Congress