Antibody Response to 3-Dose SARS-CoV-2 mRNA Vaccination in Pediatric Solid Organ Transplant Recipients
C. X. Qin1, S. R. Auerbach2, O. Charnaya1, L. A. Danziger-Isakov3, N. H. Ebel4, A. G. Feldman2, E. K. Hsu5, J. McAteer1, S. Mohammad6, E. R. Perito7, A. M. Thomas1, T. P. Chiang1, J. M. Garonzik-Wang1, D. L. Segev1, D. B. Mogul1
1Johns Hopkins University, Baltimore, MD, 2University of Colorado, Aurora, CO, 3University of Cincinnati, Cincinnati, OH, 4Stanford University, Palo Alto, CA, 5University of Washington, Seattle, WA, 6Northwestern University, Chicago, IL, 7University of California San Francisco, San Francisco, CA
Meeting: 2022 American Transplant Congress
Abstract number: 689
Keywords: Antibodies, COVID-19, Pediatric, Vaccination
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis) I
Session Type: Poster Abstract
Date: Saturday, June 4, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
*Purpose: We report the immunogenicity and safety of a third BNT162b2 vaccine in pediatric solid organ transplant recipients (pSOTRs).
*Methods: Samples from pSOTRs (12-18 years) enrolled in our multicenter, observational study (COVID-19 Antibody Testing of Recipients of Solid Organ Transplants and Patients with Chronic Diseases) who received a third vaccine (V3) were analyzed for antibodies to SARS-CoV-2 spike protein receptor-binding domain, with a positive cutoff of ≥0.8 and maximum titer of >2500 U/mL. Pre-V3 samples were 1-3 months after vaccine 2, and post-V3 were 1 month after vaccine 3.
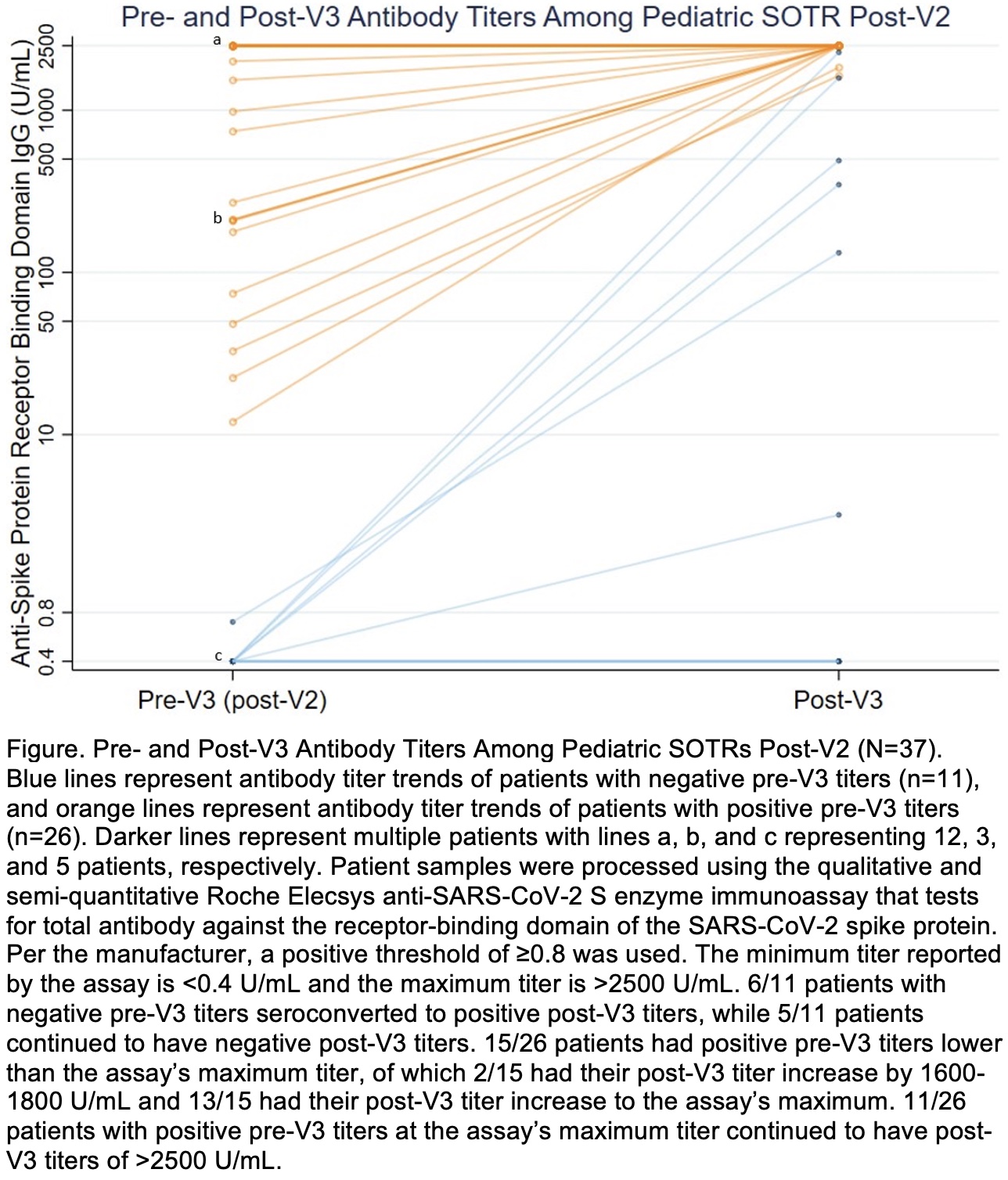
*Results: Thirty-seven pSOTRs (46% heart, 24% liver, 27% kidney, 3% multi) received V3. Median (interquartile range [IQR]) age was 15 (14-16) years; 42% were male and 78% white. pSOTRs were median (IQR) 9 (6-13) years from transplant. Four (11%) patients had prior SARS-CoV-2 infection. Antibody titers were positive in 26/37 (70%) patients pre-V3 and 32/37 (86%) post-V3 (Figure). Median (IQR) antibody titers were higher post-V3 (2500 [1581-2500] U/mL) than pre-V3 (211 [0.8-2500] U/mL) in paired analysis (p<0.001). 6/11 (55%) pSOTRs with negative pre-V3 titers seroconverted, with a post-V3 median (IQR) titer of 418 (132-1581) U/mL. Transplant within 3 years was associated with negative post-V3 titer (p=0.037). Main side effects after V3 were pain (71%) and fatigue (50%). No patients reported allergic reaction, myocarditis, or rejection. One patient tested positive for SARS-CoV-2 between vaccines 2 and 3, with negative pre- and post-V3 titers. At time of first vaccine, this patient was transplanted a year ago, treated for rejection recently, and taking 3 immunosuppression agents including an antimetabolite.
*Conclusions: In this limited cohort, 86% of pSOTRs had a positive antibody response after three SARS-CoV-2 vaccines with no adverse events. Importantly, 55% of pSOTRs with prior negative response seroconverted post-V3, and 100% of pSOTRs with positive response increased their antibody titer or remained at maximum titer. Our preliminary results suggest the benefit of a third vaccine for adolescent pSOTRs based on antibody response; larger studies are needed to assess vaccine effectiveness.
To cite this abstract in AMA style:
Qin CX, Auerbach SR, Charnaya O, Danziger-Isakov LA, Ebel NH, Feldman AG, Hsu EK, McAteer J, Mohammad S, Perito ER, Thomas AM, Chiang TP, Garonzik-Wang JM, Segev DL, Mogul DB. Antibody Response to 3-Dose SARS-CoV-2 mRNA Vaccination in Pediatric Solid Organ Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/antibody-response-to-3-dose-sars-cov-2-mrna-vaccination-in-pediatric-solid-organ-transplant-recipients/. Accessed July 6, 2025.« Back to 2022 American Transplant Congress