Antibody-Mediated Kidney Allograft Rejection (ABMR) Monitoring and Intervention Based on ABMR Subtypes – A Step Towards Precision Diagnosis and Management
1Internal Medicine, Nephrology, University of Texas Medical Branch, Galveston, TX, 2University of Texas Medical Branch, Houston, TX, 3Surgery, University of Texas Medical Branch, Galveston, TX, 4University of Texas Medical Branch, Galveston, TX
Meeting: 2022 American Transplant Congress
Abstract number: 1064
Keywords: Biopsy, IVIG, Kidney transplantation, Rejection
Topic: Clinical Science » Kidney » 44 - Kidney Acute Antibody Mediated Rejection
Session Information
Session Name: Kidney Acute Antibody Mediated Rejection
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: The monitoring of kidney allograft for early detection of antibody mediated rejection (ABMR) and subsequent intervention using traditional clinical parameters has been insufficient due to lagging response of creatinine and histologic variations on kidney biopsy. Donor-derived cell-free DNA (dd-cfDNA) is a noninvasive early marker of allograft injury. Analyses of kidney tissue using molecular microscope diagnostic system (MMDx) can enhance traditional histology interpretation. At our institute, patients are monitored for rejection at specified time intervals using serum creatinine (SCr), HLA donor specific antibodies (DSA), and dd-cfDNA. An allograft biopsy is performed in accord and sample is sent for histopathology and MMDx.
*Methods: In this single-center IRB-approved retrospective study, we included 50 patients who underwent biopsy and were diagnosed to have ABMR following above monitoring protocol. Based on histology, patients were divided into C4D positive ABMR (C+ABMR) and C4D negative ABMR (C-ABMR). The diagnostic markers (SCr, dd-cfDNA, and DSA) were assessed pre- (at the time of biopsy) and post-intervention (at last follow-up). Patients with C+ABMR were managed according to our center protocol of IV immunoglobulins (IVIG)/plasma exchange+/-Bortezomib, whereas C-ABMR patients were given IVIG. Maintenance immunosuppression was increased in both groups.
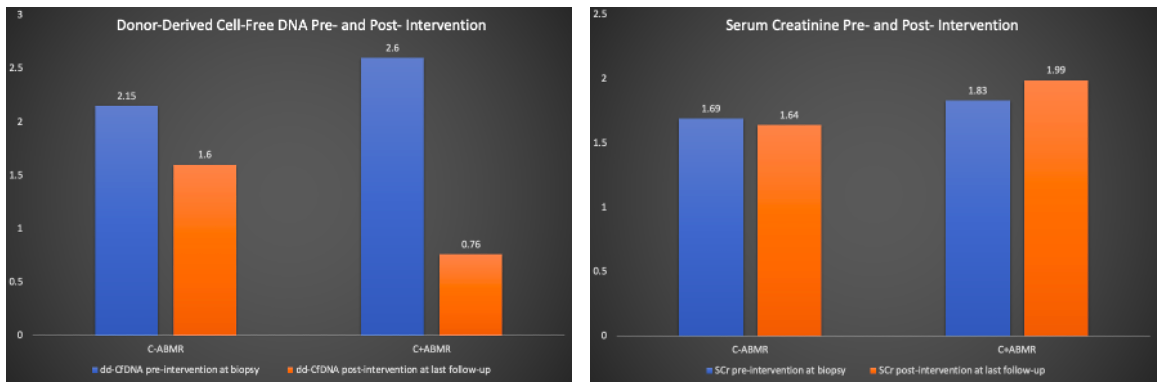
*Results: The C+ABMR group included 16 patients [median (IQR) age: 55 (42, 59) years] and C-ABMR group included 34 patients [48 (42, 63) years]. The median (IQR) follow-up duration was 504 (353, 576) days. The DSAs were positive in 50% patients in C+ABMR group and 52% patients in C-ABMR group. Among the C+ABMR and C-ABMR groups, median (IQR) dd-cfDNA and SCr are represented in Figure. Interestingly, among C-ABMR group, traditional histology was negative for ABMR in 18 patients (55%) but MMDx was positive for ABMR in 100% of patients. However, among C+ABMR group, the traditional histology was positive for ABMR in 81% of patients.
*Conclusions: The utilization of ABMR monitoring and post-intervention protocol with inclusion of dd-cfDNA and addition of MMDx to kidney biopsy has helped with early recognition, timely intervention, and individualized treatment in our practice. Inclusion of these modalities may help in improving allograft survival.
To cite this abstract in AMA style:
Rizvi A, Hussain S, Kueht M, Fair J, Gamilla-Crudo A, Mujtaba M. Antibody-Mediated Kidney Allograft Rejection (ABMR) Monitoring and Intervention Based on ABMR Subtypes – A Step Towards Precision Diagnosis and Management [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/antibody-mediated-kidney-allograft-rejection-abmr-monitoring-and-intervention-based-on-abmr-subtypes-a-step-towards-precision-diagnosis-and-management/. Accessed February 21, 2026.« Back to 2022 American Transplant Congress