Antibody and Cellular Immunity After Primary mRNA Vaccination in Adolescents with Stable Liver or Liver-Inclusive Intestine Allografts
1UPMC Children's Hospital of Pittsburgh, Pittsburgh, PA, 2Childrens Hospital of Pittsburgh of UPMC, Pittsburgh, PA
Meeting: 2022 American Transplant Congress
Abstract number: 330
Keywords: Antibodies, COVID-19, Liver transplantation, T cells
Topic: Clinical Science » Liver » 61 - Liver: Pediatrics
Session Information
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:30pm-6:40pm
Presentation Time: 6:30pm-6:40pm
Location: Hynes Room 311
*Purpose: Transplant recipients experience lower seroconversion rates after COVID-19 vaccination compared with healthy adults and have been prioritized to receive booster doses. To assess pre- and post-booster cellular and antibody immunity after COVID-19 vaccination in adolescent and young adults with liver transplants.
*Methods: In this preliminary report, we measured pre-booster frequencies of peripheral blood T-and B-cells which expressed the inflammatory marker CD154 after overnight stimulation with peptide mixtures representing the spike protein S, its S2 component which is conserved between SARS-CoV-2 and human coronaviruses, and the S1 component, which is specific to SARS-CoV-2, which also contains its receptor binding domain (RBD). Serum from each sample was assayed for anti-RBD and anti-S IgG with ELISA. OD at 450nm of 0.45 or greater implied presence of either antibody. Frequencies of monocytic and polymorphonuclear (PMN) myeloid-derived suppressor cells (M-MDSC, PMN-MDSC) were also measured with flow cytometry.
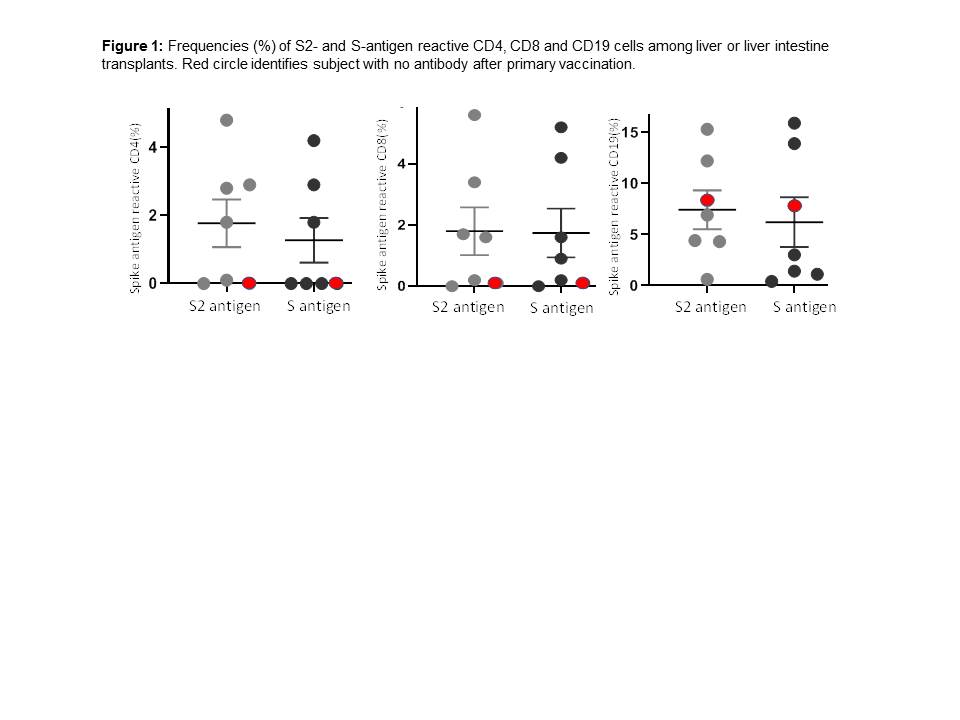
*Results: Seven LT recipients, three of whom also received intestine allografts included 6 Caucasian and one Asian. Two received mRNA1273 and 5 received BNT162b vaccination. Median age was 15 years (range 13-20) male:female distribution was 3:4. Subjects were first sampled at median 14.5years (range 10.7-18) from transplant. Median tacrolimus whole blood concentration was 4.6 ng/ml (range 2-8.4). All but one received two doses of vaccination; one opted to receive a single dose, and a liver-intestine recipient received a third dose after primary vaccination. Anti-S-IgG and anti-RBD-IgG antibodies were detected in 6/7 subjects. S2-reactive and S-reactive T-cells were detected in five and B-cells were detected in 6/7 (Figure 1). Mean+/-SEM frequencies (%) of S2 and S-reactive cells were 1.8±0.7 and 1.3±0.7 for CD4, 1.8±0.8 and 1.7±0.8 for CD8 and 7.4±1.9 and 6.2±2.4 for CD19+B-cells. In the liver-intestine recipient with no antibody response to primary vaccination, S2 and S-reactive T-cells were undetectable (Figure 1) and tacrolimus whole blood concentration of 8.4 ng/ml exceeded levels which were <6 ng/ml in the remaining subjects. A booster dose led to detectable antibodies.
*Conclusions: Most adolescent transplant recipient with a liver-inclusive allograft show robust antibody and cellular response, when vaccinated with mRNA vaccines late after transplantation. Recipients who fail to seroconvert and show impaired T-cell responses may benefit from booster doses.
To cite this abstract in AMA style:
Mazariegos G, Ashokkumar C, Soltys K, Michaels M, Bond G, Khanna A, Ningappa M, Morgan P, Sindhi R. Antibody and Cellular Immunity After Primary mRNA Vaccination in Adolescents with Stable Liver or Liver-Inclusive Intestine Allografts [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/antibody-and-cellular-immunity-after-primary-mrna-vaccination-in-adolescents-with-stable-liver-or-liver-inclusive-intestine-allografts/. Accessed February 22, 2026.« Back to 2022 American Transplant Congress