Angiotensin II-Regulated Proteins Production by Pulmonary Macrophages in Chronic Lung Allograft Dysfunction
1Toronto General Hospital Research Institute and Multi-Organ Transplant, University Health Network, Toronto, ON, Canada, 2Toronto Lung Transplant Program, University Health Network, Toronto, ON, Canada
Meeting: 2022 American Transplant Congress
Abstract number: 338
Keywords: Fibrosis, Lung transplantation, Peptides
Topic: Clinical Science » Lung » 64 - Lung: All Topics
Session Information
Session Name: Early and Late Outcomes in Lung Transplantation
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 6, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:20pm-6:30pm
Presentation Time: 6:20pm-6:30pm
Location: Hynes Room 210
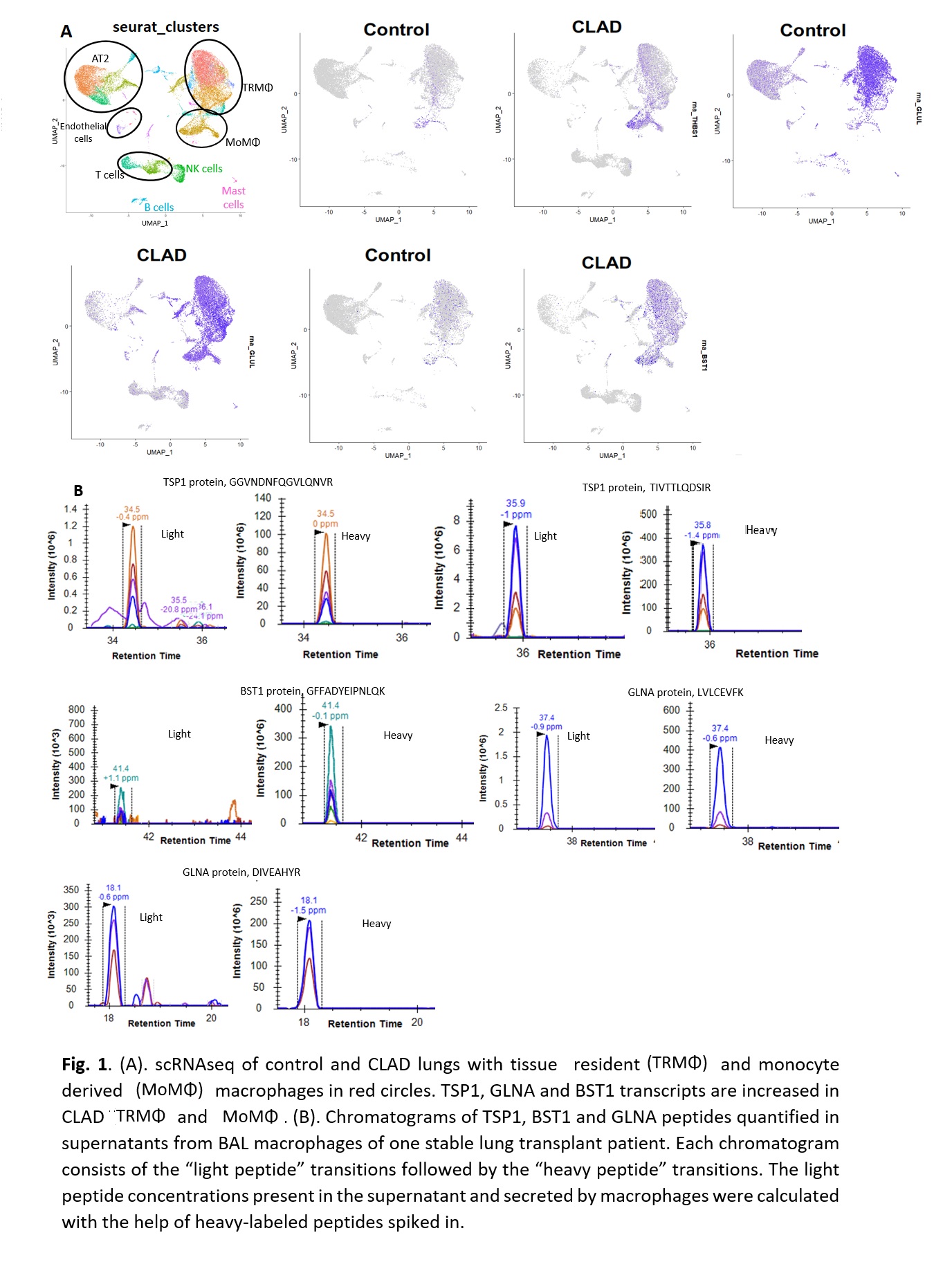
*Purpose: Chronic lung allograft dysfunction (CLAD), characterized by fibrosis, is the most important cause of mortality after lung transplantation. CLAD mechanisms remain poorly understood and effective treatments are lacking. Angiotensin II (AngII), the main effector of the renin-angiotensin system, is involved in the development of fibrosis in both kidney and lung. We identified AngII-regulated proteins in kidney cells that may play a role in fibrosis in vivo and demonstrated that 6 of these proteins (TSP1 GLNA, BST1, LAMB2, LYPLA1, RHOB, TSP1) are increased in kidney tissue and urine of patients with kidney graft fibrosis. We postulated that the renin-angiotensin system is also involved in CLAD and demonstrated increased TSP1 mRNA and TSP1 and GLNA protein in CLAD compared to control lungs, with immunostaining for both proteins localizing to macrophages (MΦ). The purpose of this study was to validate TSP1, GLNA and BST1 production by pulmonary MΦ in CLAD.
*Methods: We performed single cell RNA sequencing (scRNAseq) analysis of CLAD lungs (5) and compared to publicly available control lungs (n = 3). Lung cells isolated from bronchoalveolar lavage (BAL), mostly composed of MΦ, from a lung transplant recipient were cultured for 24 hours and the supernatant was analyzed using mass spectrometry-based targeted assays monitoring 2 peptides from TSP1, GLNA and BST1. For quantification, isotopically labeled ‘heavy’ peptides, chemically identical to the endogenously expressed peptides, were added to each sample.
*Results: Our pilot scRNAseq data revealed increased TSP1, GLNA and BST1 expression in MΦ populations (Fig 1A). Mass spectrometry assays quantified 2 peptides of TSP1 (26.3 nmol/ml) and GLNA (4.4 nmol/ml) and one peptide of BST1 (1.75 nmol/ml) in BAL MΦ culture supernatant, demonstrating that lung allograft MΦ secrete TSP1, GLNA and BST1 proteins ex vivo (Fig 1B).
*Conclusions: Our pilot data support that lung allograft MΦ express and secrete TSP1, GLNA and BST1 proteins. Future studies will investigate their potential mechanistic role in lung allograft fibrosis.
To cite this abstract in AMA style:
Farkona S, Duong A, Berra G, Moshkelgosha S, Clotet-Freixas S, Juvet S, Martinu T, Konvalinka A. Angiotensin II-Regulated Proteins Production by Pulmonary Macrophages in Chronic Lung Allograft Dysfunction [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/angiotensin-ii-regulated-proteins-production-by-pulmonary-macrophages-in-chronic-lung-allograft-dysfunction/. Accessed February 22, 2026.« Back to 2022 American Transplant Congress