An Interventional Study Using Cell-Mediated Immunity to Guide Primary Prophylaxis for CMV Infection in Organ Transplant Recipients: A Multi-Center Study
J. T. Solera1, C. Cervera2, S. Hosseini1, J. Gill3, S. Shalhoub4, J. Zaltzman5, N. Pinzon1, A. Humar1, D. Kumar1
1UHN, Toronto, ON, Canada, 2UAlberta, Edmonton, AB, Canada, 3UBC, Vancouver, BC, Canada, 4LHSC, London, ON, Canada, 5StMH, Toronto, ON, Canada
Meeting: 2022 American Transplant Congress
Abstract number: 52
Keywords: Cytomeglovirus, Interferon (IFN), Prophylaxis, T cell reactivity
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: Cytomegalovirus and other Herpes Viruses
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 3:50pm-4:00pm
Presentation Time: 3:50pm-4:00pm
Location: Hynes Ballroom B
*Purpose: There are limited interventional studies using CMV cell-mediated immunity to guide the duration of antiviral prophylaxis. We aimed to use CMV CMI to guide primary CMV prophylaxis in organ transplant recipients at high risk of CMV.
*Methods: We performed a single-arm prospective multicenter study including kidney, pancreas, liver, and heart transplant recipients who were eligible to receive antiviral prophylaxis for CMV for either (i) D+/R- serostatus or (ii) R+ status with anti-thymocyte globulin induction. CMV CMI (Quantiferon-CMV) was performed at post-transplant months 3,4,5 and prophylaxis was discontinued for a positive result but continued for a negative result up to a maximum of 6 months. Patients were monitored for CMV viremia and CMV disease up to 1 year post-transplant. The primary endpoint was CMV viremia >=1000 IU/mL.
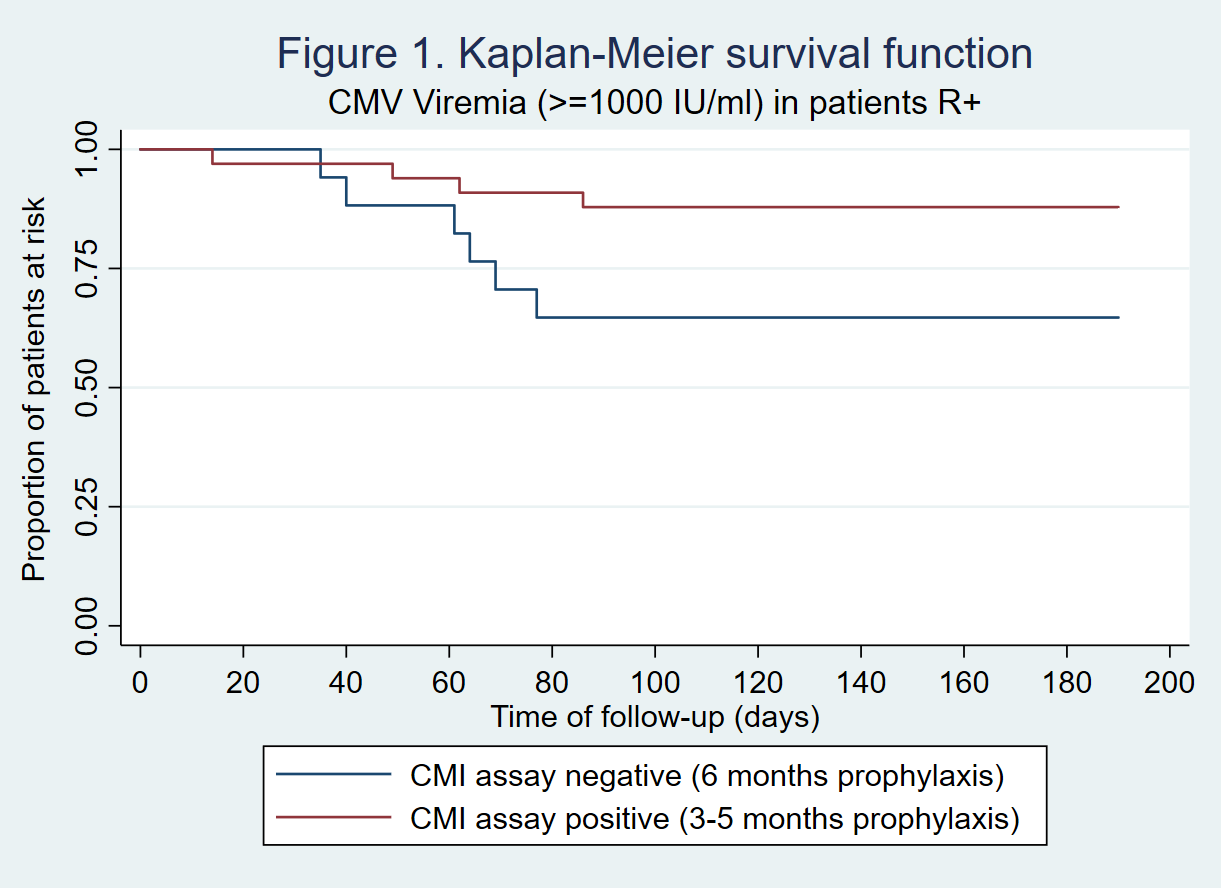
*Results: A total of 108 patients were included. 62% were males and mean age was 53.1 years (SD 13.3). Transplant types were kidney (n=89), kidney-pancreas (n=7), liver (n=10), and heart (n=2). 12% had a previous transplant. CMV serologic status was D+/R- in 48 patients (44.4%) and R+ in 60 patients (55.6%). Of these, 89 patients (82.4%) completed the follow-up period (n=39 D+/R- and n=50 R+). In the D+/R- group, only 1/39 (2.6%) had a positive CMV CMI prior to 6 months and discontinued prophylaxis early. During followup of D+/R- patients,15/38 (39.5%) CMI negative patients developed CMV viremia over 1000 IU/mL, and 5(13.2%) had CMV disease. Two patients (5.1%) had biopsy-proven rejection and 6 (15.4%) had significant leukopenia. In the R+ group, 33/50 (66%) had a positive CMI assay prior to 6 months: 28 at month 3 post-transplant, 4 at month 4, and 1 at month 5. During followup, CMV viremia >=1000 IU/mL occurred in only 4/33 (12.1%) patients that discontinued prophylaxis early and in 6/17 (35.3%) of patients that were CMI negative and continued prophylaxis till 6 months (p=0.14) (Figure 1). No R+ patient developed CMV disease. There were no significant differences in hematologic adverse effects between early discontinuation and extended 6 month prophylaxis in the R+ group.
*Conclusions: CMV CMI guided prophylaxis does not appear to be useful in D+/R- patients since the vast majority do not develop a positive CMI while on antiviral prophylaxis. However, guided prophylaxis appears useful in R+ patients who may benefit from extension of prophylaxis or close monitoring if CMI negative.
To cite this abstract in AMA style:
Solera JT, Cervera C, Hosseini S, Gill J, Shalhoub S, Zaltzman J, Pinzon N, Humar A, Kumar D. An Interventional Study Using Cell-Mediated Immunity to Guide Primary Prophylaxis for CMV Infection in Organ Transplant Recipients: A Multi-Center Study [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/an-interventional-study-using-cell-mediated-immunity-to-guide-primary-prophylaxis-for-cmv-infection-in-organ-transplant-recipients-a-multi-center-study/. Accessed March 9, 2026.« Back to 2022 American Transplant Congress