An Economical and Effective Vaccination Program in Patients Transplanted for Hepatitis B Virus Related Liver Diseases: A Prospective Study.
W. Ju, A. Yang, Q. Ren, D. Wang, Z. Guo, Y. Ma, A. Hu, Q. Tai, X. Zhu, X. He.
Organ Transplantation Center, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China.
Meeting: 2016 American Transplant Congress
Abstract number: 264
Keywords: Efficacy, Hepatitis B, Recurrence, Vaccination
Session Information
Session Name: Concurrent Session: Viral Hepatitis
Session Type: Concurrent Session
Date: Monday, June 13, 2016
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:54pm-3:06pm
Presentation Time: 2:54pm-3:06pm
Location: Ballroom A
Aims: To prospectively investigate the efficacy and safety of procedural HBV vaccination in patients transplanted for HBV related liver diseases for prevention of hepatitis B recurrence.
Methods: Patients who underwent liver transplantation for HBV related liver diseases were recruited more than 1 years after LT. Except for regular administration of anti-HBV drugs prophylaxis, hepatitis B vaccination (40[micro]g) was administered intramuscularly during months 0, 1, 2, 6 and 12 after enrolment. The results of liver function, HBsAb titers and so on were monitored during the course and HBIG would be administrated intramuscularly when HBsAb titers lower than 30 IU/L. After the completion of vaccination procedure, all subjects were withdrew of HBIG administration and followed up for 6 months. Patients whose HBsAb titer higher than 30 IU/L at the end of follow-up were considered as effective responders.
Results: 27 patients were enrolled and their HBsAb titers before vaccination was 19.87±15.38 IU/L. After follow-up, HBsAb titer in 9 responders was 57.14±22.75 IU/L. There have been no reports of HBV reactivation and severe anaphylaxis so far. The LY/EO rate (lymphocyte number/eosinophil granulocyte number) more than 20 before the vaccination may be considered as a predictor of effective responders (P=0.036). Furthermore, a longer interval between transplantation and vaccination (>600 days) may be a significant factor (P=0.059).
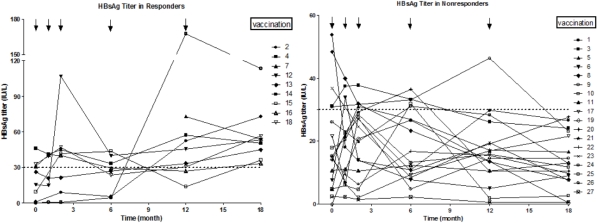
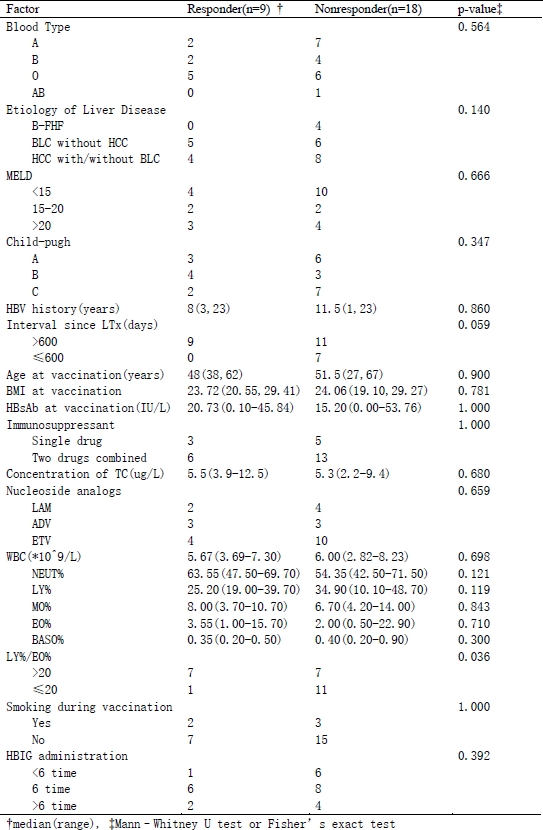
Conclusions:Active immunization is an effective, cost-saving, and safe method for prevention of HBV reactivations in patients transplanted for HBV related liver diseases. The LY/EO rate and interval time may be the potential keypoints in selecting effective responders for vaccination.
CITATION INFORMATION: Ju W, Yang A, Ren Q, Wang D, Guo Z, Ma Y, Hu A, Tai Q, Zhu X, He X. An Economical and Effective Vaccination Program in Patients Transplanted for Hepatitis B Virus Related Liver Diseases: A Prospective Study. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Ju W, Yang A, Ren Q, Wang D, Guo Z, Ma Y, Hu A, Tai Q, Zhu X, He X. An Economical and Effective Vaccination Program in Patients Transplanted for Hepatitis B Virus Related Liver Diseases: A Prospective Study. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/an-economical-and-effective-vaccination-program-in-patients-transplanted-for-hepatitis-b-virus-related-liver-diseases-a-prospective-study/. Accessed March 9, 2026.« Back to 2016 American Transplant Congress
