Adult Primary Liver Transplant Recipient Outcomes by Immunosuppression Induction Received
1Surgery, Division of Transplantation, University of Minnesota, Minneapolis, MN, 2M Health Fairview, Minneapolis, MN, 3Division of Renal Diseases and Hypertension, University of Minnesota, Minneapolis, MN, 4Division of Gastroenterology, Hepatology, and Nutrition, University of Minnesota, Minneapolis, MN
Meeting: 2020 American Transplant Congress
Abstract number: A-135
Keywords: Graft failure, Hepatitis C, Immunosuppression, Induction therapy
Session Information
Session Name: Poster Session A: Liver: Immunosuppression and Rejection
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: It has long been recognized that the highest risk of rejection of an organ is in the first few months post transplantation. To prevent acute rejection of the transplanted liver, intensive immunosuppression in the perioperative period, termed induction therapy, has been used. Induction varies widely by transplant centers, and we sought to show the impact of different induction types on recipient and graft survival.
*Methods: We analyzed the SRTR standard analysis file to evaluate all US adult primary transplant liver recipients in the MELD-allocation era. Recipients were grouped by induction type into three groups: Depleting (n=6366), Non-Depleting (n=12410), and none (steroid only, n=28317). We also divided the groups into HCV (Hepatitis C Virus) patients and non HCV patients. Variables of interest were compared using t-tests or Chi-squared tests. Patient survival and death censored graft survival were compared between induction types using Kaplan-Meier curves. All analysis was performed in R (ver. 3.6.1).
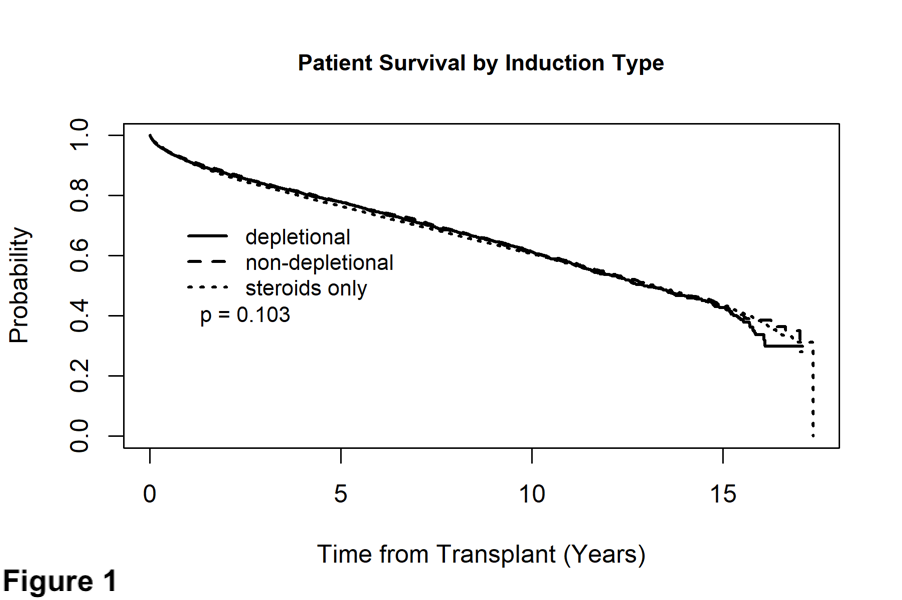
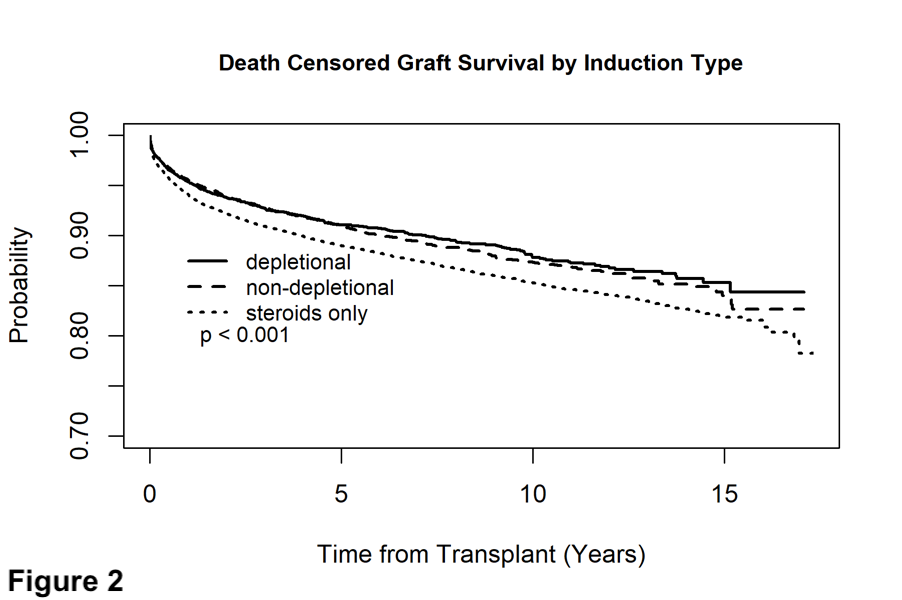
*Results: Patient survival was not influenced by induction type (Figure 1). No induction use was associated with decreased allograft survival (Figure 2), this was true in both the HCV+ and HCV- patients as well. Rejection rates at one year did not significantly differ between groups. There was no difference between groups in terms of HCC and non HCC malignancy rates at 1 year.
*Conclusions: In this large cohort of adult primary liver transplant recipients, induction with depletional and non-depletional therapy was associated with graft survival benefit. Some form of induction, whether depletional or non-depletional, may be beneficial in primary adult liver transplant recipients.
To cite this abstract in AMA style:
Shaker T, Larrieux G, Jackson S, Riad S, Pruett T, Leventhal T. Adult Primary Liver Transplant Recipient Outcomes by Immunosuppression Induction Received [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/adult-primary-liver-transplant-recipient-outcomes-by-immunosuppression-induction-received/. Accessed February 27, 2026.« Back to 2020 American Transplant Congress